Comprehensive chemoanatomical mapping, and the gonadal regulation, of seven kisspeptin neuronal populations in the mouse brain
- PMID: 40102056
- PMCID: PMC12045674
- DOI: 10.1111/jne.70019
Comprehensive chemoanatomical mapping, and the gonadal regulation, of seven kisspeptin neuronal populations in the mouse brain
Abstract
Kisspeptinergic signaling is well-established as crucial for the regulation of reproduction, but its potential broader role in brain function is less understood. This study investigates the distribution and chemotyping of kisspeptin-expressing neurons within the mouse brain. RNAscope single, dual, and multiplex in situ hybridization methods were used to assess kisspeptin mRNA (Kiss1) expression and its co-expression with other neuropeptides, excitatory and inhibitory neurotransmitter markers, and sex steroid receptors in wild-type intact and gonadectomized young adult mice. Seven distinct kisspeptin neuronal chemotypes were characterized, including two novel kisspeptin-expressing groups described for the first time, that is, the Kiss1 population in the ventral premammillary nucleus and the nucleus of the solitary tract. Kiss1 mRNA was also observed to localize in both somatic and dendritic compartments of hypothalamic neurons. High androgen receptor expression and changes in medial amygdala and septo-hypothalamic Kiss1 expression following GDX in males, but not in females, suggest a role for androgen receptors in regulating kisspeptin signaling. This study provides a detailed chemoanatomical map of kisspeptin-expressing neurons, highlighting their potential functional diversity. The discovery of a new kisspeptin-expressing group and gonadectomy-induced changes in Kiss1 expression patterns suggest broader roles for kisspeptin in brain functions beyond those of reproduction.
Keywords: Ar; Esr1; gonadectomy; neuropeptides; vesicular amino‐acid transporter.
© 2025 The Author(s). Journal of Neuroendocrinology published by John Wiley & Sons Ltd on behalf of British Society for Neuroendocrinology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

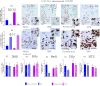
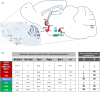
Update of
-
Comprehensive chemotyping, and the gonadal regulation, of seven kisspeptinergic neuronal populations in the mouse brain.bioRxiv [Preprint]. 2024 Nov 27:2024.07.23.604881. doi: 10.1101/2024.07.23.604881. bioRxiv. 2024. Update in: J Neuroendocrinol. 2025 May;37(5):e70019. doi: 10.1111/jne.70019. PMID: 39211104 Free PMC article. Updated. Preprint.
Similar articles
-
Kisspeptin fiber and receptor distribution analysis suggests its potential role in central sensorial processing and behavioral state control.J Neuroendocrinol. 2025 May;37(5):e70007. doi: 10.1111/jne.70007. Epub 2025 Mar 10. J Neuroendocrinol. 2025. PMID: 40065551 Free PMC article.
-
Comprehensive chemotyping, and the gonadal regulation, of seven kisspeptinergic neuronal populations in the mouse brain.bioRxiv [Preprint]. 2024 Nov 27:2024.07.23.604881. doi: 10.1101/2024.07.23.604881. bioRxiv. 2024. Update in: J Neuroendocrinol. 2025 May;37(5):e70019. doi: 10.1111/jne.70019. PMID: 39211104 Free PMC article. Updated. Preprint.
-
Anatomic distribution of kisspeptin neurons in the adult sheep amygdala: Associations with sex, estrogen receptor alpha, androgen receptor, and sexual partner preference.J Neuroendocrinol. 2025 May;37(5):e70011. doi: 10.1111/jne.70011. Epub 2025 Mar 3. J Neuroendocrinol. 2025. PMID: 40033683
-
The role of KNDy neurons in human reproductive health.Endocr J. 2024 Aug 8;71(8):733-743. doi: 10.1507/endocrj.EJ24-0006. Epub 2024 Jun 12. Endocr J. 2024. PMID: 38866494 Review.
-
Kisspeptin Neurons and Estrogen-Estrogen Receptor α Signaling: Unraveling the Mystery of Steroid Feedback System Regulating Mammalian Reproduction.Int J Mol Sci. 2021 Aug 26;22(17):9229. doi: 10.3390/ijms22179229. Int J Mol Sci. 2021. PMID: 34502135 Free PMC article. Review.
Cited by
-
Kisspeptin Administration and mRNA Expression in Adult Syrian Hamsters.Cells. 2025 Jun 29;14(13):992. doi: 10.3390/cells14130992. Cells. 2025. PMID: 40643513 Free PMC article.
-
Kisspeptin fiber and receptor distribution analysis suggests its potential role in central sensorial processing and behavioral state control.J Neuroendocrinol. 2025 May;37(5):e70007. doi: 10.1111/jne.70007. Epub 2025 Mar 10. J Neuroendocrinol. 2025. PMID: 40065551 Free PMC article.
References
-
- Kotani M, Detheux M, Vandenbogaerde A, et al. The metastasis suppressor gene KiSS‐1 encodes kisspeptins, the natural ligands of the orphan G protein‐coupled receptor GPR54. J Biol Chem. 2001;276(37):34631‐34636. - PubMed
-
- Makri A, Pissimissis N, Lembessis P, Polychronakos C, Koutsilieris M. The kisspeptin (KiSS‐1)/GPR54 system in cancer biology. Cancer Treat Rev. 2008;34(8):682‐692. - PubMed
-
- Lee JH, Miele ME, Hicks DJ, et al. KiSS‐1, a novel human malignant melanoma metastasis‐suppressor gene. J Natl Cancer Inst. 1996;88(23):1731‐1737. - PubMed
-
- Lee DK, Nguyen T, O'Neill GP, et al. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;446(1):103‐107. - PubMed
-
- Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin‐releasing hormone secretion. Endocrinology. 2006;147(3):1154‐1158. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

