From DNA-Encoded Library Screening to AM-9747: An MTA-Cooperative PRMT5 Inhibitor with Potent Oral In Vivo Efficacy
- PMID: 40102181
- PMCID: PMC11956014
- DOI: 10.1021/acs.jmedchem.4c03101
From DNA-Encoded Library Screening to AM-9747: An MTA-Cooperative PRMT5 Inhibitor with Potent Oral In Vivo Efficacy
Abstract
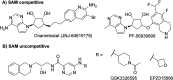
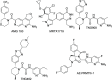
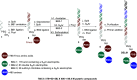
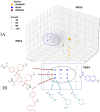
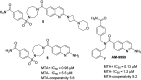
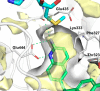
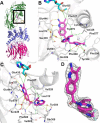
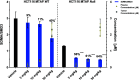
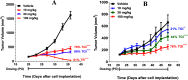
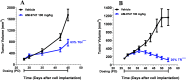
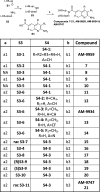
Inhibition of the methyltransferase enzyme PRMT5 by MTA accumulation is a vulnerability of MTAP-deleted cancers. Herein, we report the discovery and optimization of a quinolin-2-amine DEL hit that cooperatively binds PRMT5:MEP50 and MTA to generate a catalytically inhibited ternary complex. X-ray crystallography confirms quinolin-2-amine binding of PRMT5 glutamate-444, while simultaneously exhibiting a hydrophobic interaction with MTA. Lead optimization produced AM-9747, which selectively inhibits PRMT5-directed symmetric dimethylation of arginine residues of proteins, leading to a potent reduction of cell viability in MTAP-del cells compared to MTAP-WT cells. Once-daily oral dosing of AM-9747 in mouse xenografts is well tolerated, displaying a robust and dose-dependent inhibition of symmetric dimethylation of arginine in MTAP-del tumor-xenografts and significant concomitant tumor growth inhibition without any significant effect on MTAP-WT tumor xenografts.
Conflict of interest statement
The authors declare the following competing financial interest(s): All authors were employed with Amgen Inc when the research was conducted.
Figures


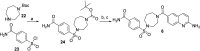
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

