Development and validation of cancer-specific survival prediction nomogram for patients with T4 stage colon cancer after surgical resection: a population-based study
- PMID: 40102264
- PMCID: PMC11920356
- DOI: 10.1007/s00384-025-04856-3
Development and validation of cancer-specific survival prediction nomogram for patients with T4 stage colon cancer after surgical resection: a population-based study
Abstract
Purpose: The increasing incidence of colorectal cancer has coincided with a rise in T4 stage colon cancer (CC), yet research on its prognosis remains limited. This study aimed to identify risk factors and develop a nomogram to predict cancer-specific survival (CSS), optimizing treatment strategies for different subgroups.
Methods: Using data from the from the Surveillance, Epidemiology, and End Results (SEER) database, we identified risk factors in T4 stage CC patients and created a nomogram to predict CSS. Patients were divided into low- and high-risk groups, and the nomogram was validated. Propensity score matching was used to evaluate the benefits of various therapies across subgroups.
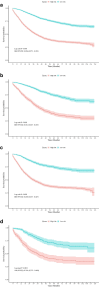
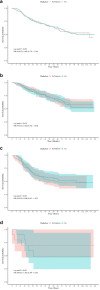
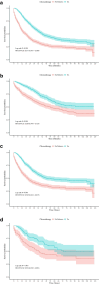
Results: Independent risk factors, including T stage, N stage, tumor grade, age, and therapy sequence, were identified through Cox regression analyses and incorporated into the nomogram. The nomogram outperformed the American Joint Committee on Cancer (AJCC) 7th staging system, with a Concordance-index of 0.77 in both training and validation sets. The receiver operating characteristic curves showed area under the curve values of 0.81, 0.77, and 0.75 for 1-, 3-, and 5-year CSS, respectively. Calibration plots confirmed strong alignment between predicted and actual outcomes, and decision curve analysis highlighted the nomogram's superior clinical utility. Chemotherapy significantly improved CSS, while radiation did not. Adjuvant therapy was particularly beneficial in high-risk groups.
Conclusion: This study offered a thorough prognostic analysis of T4 stage colon cancer patients and developed nomograms for predicting CSS. Subgroup analyses highlight the potential benefits of various treatment options.
Keywords: Adjuvant therapy; Colon cancer; Nomogram; Surveillance, Epidemiology, and End Results; T4 stage.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Competing interests: The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

