Engineering the bone reconstruction surgery: the case of the masquelet-induced membrane technique
- PMID: 40102268
- PMCID: PMC11919993
- DOI: 10.1007/s00068-025-02815-9
Engineering the bone reconstruction surgery: the case of the masquelet-induced membrane technique
Abstract
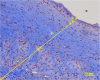
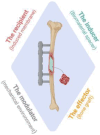
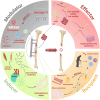
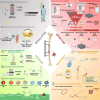
The reconstruction of large bone defects remains challenging for orthopedic surgeons. Autologous bone grafts (ABGs) are the gold standard treatment for limited size defects, but larger bone defects (> 5 cm) require the use of more sophisticated techniques, such as the Masquelet technique. Over the last three decades, the Masquelet or induced membrane technique (IMT) has become increasingly popular as it does not require high-precision microsurgery skills and the time taken to achieve bone consolidation is independent of the length of the defect. IMT is a two-stage procedure. In the first stage, a polymethylmethacrylate (PMMA) cement spacer is implanted into the bone lesion and a physiological immune reaction initiates the formation of a fibrotic induced membrane (IM) with both angiogenic and osteogenic properties. The second stage, performed several weeks later, involves removal of the spacer followed by the implantation of a standard ABG in the preserved IM cavity for subsequent bone repair. In this extensive review, we explain how the success of this surgical procedure can be attributed to the synergy of four key components: the inducer (the PMMA cement), the recipient (the IM), the effector (the bone graft) and the modulator (the mechanical environment). Conversely, we then explain how each key component can contribute to the failure of such treatment. Finally, we discuss existing or emerging innovative and biotechnology-oriented strategies for optimizing surgical outcome with respect to the four components of IMT described above.
Keywords: Bone repair; Foreign body reaction; Induced membrane; Masquelet; Orthopedic surgery.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

