An Outbreak of Synthetic Cannabinoid-Adulterated Tianeptine Products in New Jersey - Case Series
- PMID: 40102319
- PMCID: PMC11933608
- DOI: 10.1007/s13181-025-01068-7
An Outbreak of Synthetic Cannabinoid-Adulterated Tianeptine Products in New Jersey - Case Series
Abstract
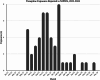
Background: Tianeptine, an atypical antidepressant not approved in the United States, is readily purchased from unregulated markets such as the internet and gas stations. We became aware of a cluster of 34 patients in New Jersey who became ill following ingestion of the tianeptine containing-product Neptune's Fix, the rate of which (4.6 cases per month) far exceeded the background rate for this substance of 0.5 cases per year.
Methods: We retrospectively identified tianeptine exposures reported to the New Jersey Poison Information and Education System (NJPIES) prior to June 2023 to determine the background rate of tianeptine exposure. From June 2023- February 2024 we prospectively surveilled tianeptine exposures reported to NJPIES, recorded demographic and clinical information, and recruited samples for testing. Six samples of the ingested products were obtained and analyzed using gas chromatography mass spectrometry (GC-MS) and liquid chromatography quadrupole time-of-flight mass spectrometry (LC-QTOF-MS). Whole blood samples from two patients were tested for tianeptine and synthetic cannabinoids.
Results: During the period of interest, NJPIES received 41 exposure calls, with 37 reporting acute toxicity in 34 unique patients, two reporting chronic tianeptine use, and two reporting withdrawal. Among the 37 exposures resulting in acute toxicity, commonly reported effects included altered mental status, tachycardia, hypotension, and seizures. 43% (n = 16) were intubated, and 65% (n = 24) were admitted to the ICU. Analytical testing of six samples identified variable product composition, containing various xenobiotics including tianeptine, kava alkaloids, natural cannabinoids, and the synthetic cannabinoids MDMB-4en-PINACA and ADB-4en-PINACA. MDMB-4en-PINACA was detected in one of the two patient blood specimens.
Conclusions: These cases represent a marked increase in tianeptine exposures compared with the poison center's historical average. Analytical testing revealed variable product composition, including the presence of synthetic cannabinoids. Clinicians should be aware that tianeptine containing products are widely available, unregulated, and can be adulterated.
Keywords: Cannabinoid; Outbreak; Tianeptine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Previous Presentation: Data in this manuscript were previously presented at ACMT’s Annual Scientific Meeting, Washington, D.C., 2024. Conflict of Intrest: DC reports receipt of honoraria from St. Peter’s University Hospital (New Jersey) for Pediatric Grand Rounds presentation, payment from the District Attorney of New York for expert testimony in a criminal case, support to Poison Center to defray costs of professional conference travel from the New Jersey Department of Health, and membership on the American College of Medical Toxicology Board of Trustees (through January 2023). HG reports receipt of honoraria from UpToDate for authored content and reimbursement from the Department of Emergency Medicine, Rutgers University, for travel to meetings. AK reports funding from the National Institute of Justice, U.S. Department of Justice, for sample analysis and salary. TC, CC, and AS reports receipt of reimbursement for travel expenses and conference fees from Rutgers University. SW, LN, BR, OH, and BL declare that they have no conflict of interest.
Figures
References
-
- Alamo C, García-Garcia P, Lopez-Muñoz F, Zaragozá C. Tianeptine, an atypical Pharmacological approach to depression. Rev Psiquiatr Salud Ment Engl Ed. 2019;12:170–86. - PubMed
-
- US Food & Drug Administration. Tianeptine Products Linked to Serious Harm, Overdoses, Death [Internet]. FDA. FDA. 2022 [cited 2024 Jan 5]. Available from: https://www.fda.gov/consumers/consumer-updates/tianeptine-products-linke...
-
- US Food & Drug Administration. Tianeptine in Dietary Supplements [Internet]. FDA. FDA. 2023 [cited 2024 Jan 5]. Available from: https://www.fda.gov/food/dietary-supplement-ingredient-directory/tianept...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous