Decreased dihydroartemisinin-piperaquine protection against recurrent malaria associated with Plasmodium falciparum plasmepsin 3 copy number variation in Africa
- PMID: 40102390
- PMCID: PMC11920258
- DOI: 10.1038/s41467-025-57726-5
Decreased dihydroartemisinin-piperaquine protection against recurrent malaria associated with Plasmodium falciparum plasmepsin 3 copy number variation in Africa
Abstract
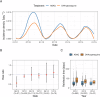
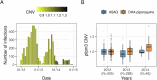
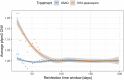
Dihydroartemisinin-piperaquine (DHA-PPQ) is being recommended in Africa for the management of uncomplicated Plasmodium falciparum malaria and for chemoprevention strategies, based on the ability of piperaquine to delay re-infections. Although therapeutic resistance to piperaquine has been linked to increased copy number in plasmepsin-coding parasite genes (pfpm), their effect on the duration of the post-treatment prophylactic period remains unclear. Here, we retrospectively analyzed data from a randomized clinical trial, where patients received either DHA-PPQ or artesunate-amodiaquine for recurrent malaria episodes over two years. We observed an increase in the relative risk of re-infection among patients receiving DHA-PPQ compared to artesunate-amodiaquine after the first malaria season. This was driven by shorter average times to reinfection and coincided with an increased frequency of infections comprising pfpm3 multi-copy parasites. The decline in post-treatment protection of DHA-PPQ upon repeated use in a high transmission setting raises concerns for its wider use for chemopreventive strategies in Africa.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: We declare no competing interests.
Figures
References
-
- World Health Organization. World malaria report 2024: addressing inequity in the global malaria response. (2024).
-
- World Health Organization. Mass Drug Administration for Falciparum Malaria. (2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

