Cytosolic nucleic acid sensing as driver of critical illness: mechanisms and advances in therapy
- PMID: 40102400
- PMCID: PMC11920230
- DOI: 10.1038/s41392-025-02174-2
Cytosolic nucleic acid sensing as driver of critical illness: mechanisms and advances in therapy
Abstract
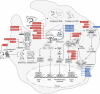
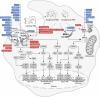
Nucleic acids from both self- and non-self-sources act as vital danger signals that trigger immune responses. Critical illnesses such as acute respiratory distress syndrome, sepsis, trauma and ischemia lead to the aberrant cytosolic accumulation and massive release of nucleic acids that are detected by antiviral innate immune receptors in the endosome or cytosol. Activation of receptors for deoxyribonucleic acids and ribonucleic acids triggers inflammation, a major contributor to morbidity and mortality in critically ill patients. In the past decade, there has been growing recognition of the therapeutic potential of targeting nucleic acid sensing in critical care. This review summarizes current knowledge of nucleic acid sensing in acute respiratory distress syndrome, sepsis, trauma and ischemia. Given the extensive research on nucleic acid sensing in common pathological conditions like cancer, autoimmune disorders, metabolic disorders and aging, we provide a comprehensive summary of nucleic acid sensing beyond critical illness to offer insights that may inform its role in critical conditions. Additionally, we discuss potential therapeutic strategies that specifically target nucleic acid sensing. By examining nucleic acid sources, sensor activation and function, as well as the impact of regulating these pathways across various acute diseases, we highlight the driving role of nucleic acid sensing in critical illness.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Targeting Cytosolic Nucleic Acid-Sensing Pathways for Cancer Immunotherapies.Front Immunol. 2018 Apr 9;9:711. doi: 10.3389/fimmu.2018.00711. eCollection 2018. Front Immunol. 2018. PMID: 29686682 Free PMC article. Review.
-
Innate immune sensing and signaling of cytosolic nucleic acids.Annu Rev Immunol. 2014;32:461-88. doi: 10.1146/annurev-immunol-032713-120156. Annu Rev Immunol. 2014. PMID: 24655297 Review.
-
Cytosolic Nucleic Acid Sensors in Inflammatory and Autoimmune Disorders.Int Rev Cell Mol Biol. 2019;344:215-253. doi: 10.1016/bs.ircmb.2018.10.002. Epub 2018 Dec 3. Int Rev Cell Mol Biol. 2019. PMID: 30798989 Review.
-
Cytoplasmic Mechanisms of Recognition and Defense of Microbial Nucleic Acids.Annu Rev Cell Dev Biol. 2018 Oct 6;34:357-379. doi: 10.1146/annurev-cellbio-100617-062903. Epub 2018 Aug 10. Annu Rev Cell Dev Biol. 2018. PMID: 30095291 Review.
-
Detection of Microbial Infections Through Innate Immune Sensing of Nucleic Acids.Annu Rev Microbiol. 2018 Sep 8;72:447-478. doi: 10.1146/annurev-micro-102215-095605. Annu Rev Microbiol. 2018. PMID: 30200854 Review.
Cited by
-
A feedback loop between DNA damage, genomic instability, and cytoplasmic DNA sensing contributes to cytokine production in COVID-19.Arch Virol. 2025 Aug 11;170(9):192. doi: 10.1007/s00705-025-06383-6. Arch Virol. 2025. PMID: 40788382 Free PMC article. Review.
References
-
- McWhirter, S. M. & Jefferies, C. A. Nucleic acid sensors as therapeutic targets for human disease. Immunity53, 78–97 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

