Monounsaturated fatty acids promote cancer radioresistance by inhibiting ferroptosis through ACSL3
- PMID: 40102409
- PMCID: PMC11920413
- DOI: 10.1038/s41419-025-07516-0
Monounsaturated fatty acids promote cancer radioresistance by inhibiting ferroptosis through ACSL3
Erratum in
-
Correction: Monounsaturated fatty acids promote cancer radioresistance by inhibiting ferroptosis through ACSL3.Cell Death Dis. 2025 Jun 3;16(1):430. doi: 10.1038/s41419-025-07726-6. Cell Death Dis. 2025. PMID: 40461470 Free PMC article. No abstract available.
Abstract
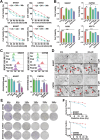
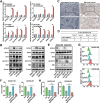
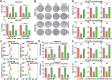
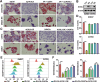
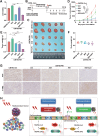
Radioresistance is a major challenge in tumor radiotherapy and involves in a mixture of cellular events, including ferroptosis, a new type of programmed cell death characterized by the excess accumulation of iron-dependent lipid peroxides. In the present study, we observed that surviving cancer tissues and cells after radiotherapy had significantly greater glutathione to oxidized glutathione (GSH/GSSG) ratios and lower lipid reactive oxygen species (ROS) and malondialdehyde (MDA) levels than nonirradiated tumors and cells. Untargeted lipidomic analyses revealed that oleic acid (OA) and palmitoleic acid (POA) were the most significantly upregulated unsaturated fatty acids in irradiated surviving cancer cells compared with those in control cancer cells irradiated with IR. Both OA and POA could protect cancer cells from the killing effects of the ferroptosis inducer erastin and RSL3, and OA had a stronger protective effect than POA, resulting in lower lipid ROS production than POA. Mechanistically, OA protected cells from ferroptosis caused by the accumulation of polyunsaturated fatty acid-containing phospholipids in an ACSL3-dependent manner. A mouse model demonstrated that ACSL3 knockdown combined with imidazole ketone erastin synergistically enhanced antitumor effects in radiation-resistant tumors in vivo. Our study reveals previously undiscovered associations between radiation and fatty acid metabolism and ferroptosis, providing a novel treatment strategy for overcoming cancer radioresistance.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics: The present study was approved by the Clinical Research Ethics Committees of the participating institutions. Human primary rectal cancer tissues from patients receiving neoadjuvant RT (before and after RT) were collected from Affiliated Hospital of Jiangnan University with informed consent. The study was approved by the Clinical Research Ethics Committees of Affiliated Hospital of Jiangnan University [LS2021104]. All animal experiments were performed in accordance with the relevant institutional and national guidelines and the regulations of Jiangnan University Medical Experimental Animal Care Commission (JN. No20230228c0480515).
Figures


References
-
- Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18:280–96. - PubMed
-
- Gebicki JM, Du J, Collins J, Tweeddale H. Peroxidation of proteins and lipids in suspensions of liposomes, in blood serum, and in mouse myeloma cells. Acta Biochim Pol. 2000;47:901–11. - PubMed
-
- Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–22. - PubMed
MeSH terms
Substances
Grants and funding
- 82473060/National Natural Science Foundation of China (National Science Foundation of China)
- 82173063/National Natural Science Foundation of China (National Science Foundation of China)
- 82472939/National Natural Science Foundation of China (National Science Foundation of China)
- 82173193/National Natural Science Foundation of China (National Science Foundation of China)
- No. BK20211033/Natural Science Foundation of Jiangsu Province (Jiangsu Provincial Natural Science Foundation)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

