Bone marrow adipogenic lineage precursors are the major regulator of bone resorption in adult mice
- PMID: 40102423
- PMCID: PMC11920254
- DOI: 10.1038/s41413-025-00405-4
Bone marrow adipogenic lineage precursors are the major regulator of bone resorption in adult mice
Abstract
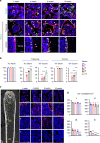
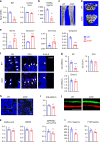
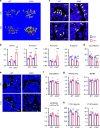
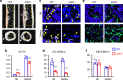
Bone resorption by osteoclasts is a critical step in bone remodeling, a process important for maintaining bone homeostasis and repairing injured bone. We previously identified a bone marrow mesenchymal subpopulation, marrow adipogenic lineage precursors (MALPs), and showed that its production of RANKL stimulates bone resorption in young mice using Adipoq-Cre. To exclude developmental defects and to investigate the role of MALPs-derived RANKL in adult bone, we generated inducible reporter mice (Adipoq-CreER Tomato) and RANKL deficient mice (Adipoq-CreER RANKLflox/flox, iCKO). Single cell-RNA sequencing data analysis and lineage tracing revealed that Adipoq+ cells contain not only MALPs but also some mesenchymal progenitors capable of osteogenic differentiation. In situ hybridization showed that RANKL mRNA is only detected in MALPs, but not in osteogenic cells. RANKL deficiency in MALPs induced at 3 months of age rapidly increased trabecular bone mass in long bones as well as vertebrae due to diminished bone resorption but had no effect on the cortical bone. Ovariectomy (OVX) induced trabecular bone loss at both sites. RANKL depletion either before OVX or at 6 weeks post OVX protected and restored trabecular bone mass. Furthermore, bone healing after drill-hole injury was delayed in iCKO mice. Together, our findings demonstrate that MALPs play a dominant role in controlling trabecular bone resorption and that RANKL from MALPs is essential for trabecular bone turnover in adult bone homeostasis, postmenopausal bone loss, and injury repair.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Update of
-
Bone marrow adipogenic lineage precursors are the major regulator of bone resorption in adult mice.Res Sq [Preprint]. 2024 Aug 28:rs.3.rs-4809633. doi: 10.21203/rs.3.rs-4809633/v1. Res Sq. 2024. Update in: Bone Res. 2025 Mar 19;13(1):39. doi: 10.1038/s41413-025-00405-4. PMID: 39257979 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- P30AR069619/Penn | Perelman School of Medicine, University of Pennsylvania (Perelman School of Medicine at the University of Pennsylvania)
- R01 AG069401/AG/NIA NIH HHS/United States
- U54HL165442/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01AG069401/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- U54 HL165442/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

