Soy protein selectively accumulates formaldehyde
- PMID: 40102440
- PMCID: PMC11920237
- DOI: 10.1038/s41598-025-92743-w
Soy protein selectively accumulates formaldehyde
Abstract
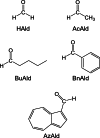
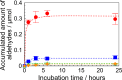
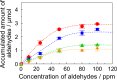
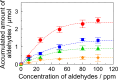
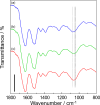
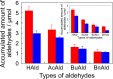
Soy protein (SP) is easily obtained from defatted soybeans that have had soybean oil removed. Therefore, the materials consisting of soy protein are not only environmentally benign but also sustainable materials. We prepared the SP - GPTMS composite materials by mixing the SP and a silane coupling reagent, 3-glycidoxypropyltrimethoxysilane (GPTMS), and demonstrated the accumulation of various aldehydes, such as formaldehyde (HAld), acetaldehyde (AcAld), butyl aldehyde (BuAld), and benzaldehyde (BnAld), by the SP - GPTMS composite materials. As a result, when the composite materials were incubated in an aqueous multi-component solution containing four aldehydes, these materials effectively accumulated the aldehydes. The accumulated amounts of the aldehydes were BnAld < BuAld < AcAld < HAld and the amount of HAld was three times higher than that of BnAld, which had the lowest accumulated amount. These results suggested that the SP - GPTMS composite materials indicated a molecular selectivity for HAld. In addition, the accumulated amounts of HAld further increased under acidic conditions. Furthermore, according to the IR measurements, the HAld-accumulated SP - GPTMS composite materials showed the formation of Schiff base bonds. Therefore, the molecular selectivity of HAld in the SP - GPTMS composite material was due to the high electrophilicity of HAld and the low steric hindrance.
Keywords: Aldehydes; Formaldehyde; Selective accumulation; Soy protein; Sustainable bio-resources.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




References
-
- Zhang, L. Formaldehyde: exposure, toxicity and health effectsThe Royal Society of Chemistry, (2018). 10.1039/9781788010269-00001
-
- McLaughlin, J. K. Formaldehyde and cancer: a critical review. Int. Arch. Occup. Environ. Health. 66, 295–301 (1994). - PubMed
-
- Barbour, A. K. et al. Air Pollution and Health (The Royal Society of Chemistry, 1998). 10.1039/9781847550095
-
- Sick building syndrome, World Health Organization Regional Office for Europe. https://www.aivc.org/sites/default/files/members_area/medias/pdf/Inive/E... (accessed 2024-01-01).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

