Complement-mediated enhancement of SARS-CoV-2 antibody neutralisation potency in vaccinated individuals
- PMID: 40102474
- PMCID: PMC11920438
- DOI: 10.1038/s41467-025-57947-8
Complement-mediated enhancement of SARS-CoV-2 antibody neutralisation potency in vaccinated individuals
Abstract
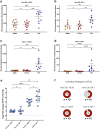
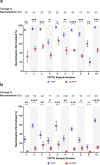
With the continued emergence of SARS-CoV-2 variants and concerns of waning immunity, there is a need for better defined correlates of protection to aid future vaccine and therapeutic developments. Whilst neutralising antibody titres are associated with protection, these are typically determined in the absence of the complement system, which has the potential to enhance neutralisation titres and strengthen correlates with protection in vivo. Here we show that replenishment of the complement system in neutralisation assays can significantly enhance neutralisation titres, with up to an ~83-fold increase in neutralisation of the BA.1.1.529 strain using cross-reactive sera from vaccination against the ancestral strain. The magnitude of enhancement significantly varies between individuals, viral strains (wild-type/VIC01 and Omicron/BA.1), and cell lines (Vero E6 and Calu-3), and is abrogated following heat-inactivation of the complement source. Utilising ACE2 competition assays, we show that the mechanism of action is partially mediated by reducing ACE2-spike interactions. Through the addition of compstatin (a C3 inhibitor) to live virus neutralisation assays, the complement protein C3 is shown to be required for maximum efficiency. These findings further our understanding of SARS-CoV-2 immunity and neutralisation, with implications for protection against emerging variants and assessing future vaccine and therapeutic developments.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Wilson, J. A. et al. Epitopes involved in antibody-mediated protection from Ebola virus. Science287, 1664–1666 (2000). - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

