Anticancer drugs targeting topoisomerase II for antifungal treatment
- PMID: 40102495
- PMCID: PMC11920057
- DOI: 10.1038/s41598-025-93863-z
Anticancer drugs targeting topoisomerase II for antifungal treatment
Abstract
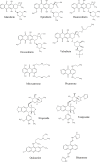
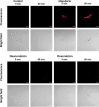
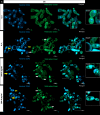
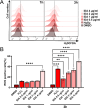
Fungal topoisomerase II (TopoII) has been identified as essential for viability. Thus, our research aimed to investigate the potential of fungal TopoII as a novel target for antifungal chemotherapy. We conducted studies on eleventh antitumor compounds targeting human topoisomerase II, either approved by the U.S. Food and Drug Administration (FDA) or currently under clinical trials to evaluate their potential for use in other therapeutic applications. While most of the compounds we analyzed are potent inhibitors of yeast TopoII, only a few exhibited antifungal activity. Idarubicin emerged as the most potent compound effectively inhibiting the growth of five reference fungal strains as well as clinical Candida glabrata fluconazole-resistant cells. Antifungal activity of this compound corresponded with its very high yeast TopoII inhibitory effectiveness. Additionally, idarubicin ability to be effectively accumulated into fungal cells is crucial for yeast TopoII targeting. Idarubicin, epirubicin, and bisantrene appeared to be even more effective inhibitors of yeast enzyme than its human counterpart. In fungal cells idarubicin exhibited a multifaceted mechanisms of action, including nuclear DNA fragmentation, disruption of mitochondrial network architecture and mitochondrial DNA aggregation as well as oxidative stress induction. Our results indicate that fungal topoisomerase II targeting is worth considering in antifungal treatment and the reported drugs may serve as a starting point for the reinnovation of a new molecule.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
A new 1-nitro-9-aminoacridine derivative targeting yeast topoisomerase II able to overcome fluconazole-resistance.Bioorg Med Chem Lett. 2021 Mar 1;35:127815. doi: 10.1016/j.bmcl.2021.127815. Epub 2021 Jan 21. Bioorg Med Chem Lett. 2021. PMID: 33486051
-
Targeting topoisomerase II with trypthantrin derivatives: Discovery of 7-((2-(dimethylamino)ethyl)amino)indolo[2,1-b]quinazoline-6,12-dione as an antiproliferative agent and to treat cancer.Eur J Med Chem. 2020 Sep 15;202:112504. doi: 10.1016/j.ejmech.2020.112504. Epub 2020 Jul 4. Eur J Med Chem. 2020. PMID: 32712536
-
Inhibitors of DNA topoisomerases I and II applied to Candida dubliniensis reduce growth, viability, the generation of petite mutants and toxicity, while acting synergistically with fluconazole.FEMS Yeast Res. 2021 Apr 27;21(3):foab023. doi: 10.1093/femsyr/foab023. FEMS Yeast Res. 2021. PMID: 33837766
-
Multiple Topoisomerase I (TopoI), Topoisomerase II (TopoII) and Tyrosyl-DNA Phosphodiesterase (TDP) inhibitors in the development of anticancer drugs.Eur J Pharm Sci. 2021 Jan 1;156:105594. doi: 10.1016/j.ejps.2020.105594. Epub 2020 Oct 12. Eur J Pharm Sci. 2021. PMID: 33059042 Review.
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
References
-
- WHO fungal priority pathogens list to guide research, development and public health action. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO. Licence: CC BY-NC-SA 3.0 IGO.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

