Utility and cost-effectiveness of LiverMultiScan for MASLD diagnosis: a real-world multi-national randomised clinical trial
- PMID: 40102528
- PMCID: PMC11920111
- DOI: 10.1038/s43856-025-00796-9
Utility and cost-effectiveness of LiverMultiScan for MASLD diagnosis: a real-world multi-national randomised clinical trial
Abstract
Background: Increasing prevalence of metabolic dysfunction-associated liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) poses a growing healthcare burden. Noninvasive diagnostic tools to replace liver biopsy are urgently needed. We investigated the utility and cost-effectiveness of including multiparametric magnetic resonance imaging (mpMRI) to the management of adults with suspected MASLD multi-nationally.
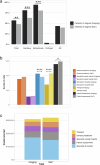
Methods: RADIcAL-1, a 1:1 randomised controlled trial (standard-of-care [SoC] vs. imaging arm [IA; SoC+mpMRI]) included 802 participants from Germany, Netherlands, Portugal and UK. Wilcoxon-rank tests were used to compare access to healthcare practitioners, patient assessments and proportion of patients with a diagnosis (%diagnosis). Liver fat and disease activity (corrected T1 [cT1]) were used to identify patients not requiring biopsy in the imaging arm. Primary endpoint was mpMRI cost-effectiveness and improvement in resource use (visits avoided) using mpMRI.
Results: mpMRI is cost-effective with an ICER of €4968/QALY gained. 403 were randomised to IA and 399 to SoC. SoC has significantly more specialist appointments (p = 0.015) and patient assessments (p < 0.001). Across all involved hospitals, %diagnosis is significantly higher in the imaging arm (p = 0.0012). cT1 correctly classifies 50% of patients without MASH with fibrosis and can avoid biopsy. Including all costs, the imaging arm incurs higher short-term per-patient healthcare expenditure compared to the SoC arm (€1,300 vs. €830).
Conclusion: Adding mpMRI to SoC for the management of adults with suspected MASLD multi-nationally is cost-effective, enhances rate of diagnosis multi-nationally and increases rate of diagnosis without increasing other liver-related health care resource use. Due to the need for standardisation of SoC, widespread use can support optimisation of the MASLD clinical pathway and improve long-term patient management.
Plain language summary
Steatotic liver disease is a global health problem which needs better diagnostic pathways. Here, we compared the number of doctor visits, the speed of diagnosis, and whether the cost of adding an MRI scan (LiverMultiScan) is justified by the improvement in patients’ quality of life across Germany, the Netherlands, Portugal, and the UK. Findings show that using an MRI scan is a safer and pain-free alternative that can help doctors diagnose more people with fewer visits, making it a cost-effective option. These results are important because they show that using the MRI scan is affordable and effective enough to be recommended as it can make diagnosing liver disease faster, more accurate, and less invasive.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare the following competing interests: MF, ES, CB, and RB are employees of Perspectum Ltd. DT is a consultant for Perspectum Ltd. JEC is a student at University College London doing a Knowledge Transfer Program (KTP) with Perspectum Ltd. All other co-authors declare no competing interests relevant to this work. The funder did not have a role in the study design, data analysis or manuscript preparation. The views expressed, are those of the author(s) and not necessarily those of the funding body (European Union’s Horizon 2020).
Figures

References
-
- Zelber-Sagi, S., Bugianesi, E., Newsome, P. & Ratziu, V. Obesity Is Feeding the Rise in Non-Alcoholic Fatty Liver Disease (NAFLD) across Europe (2019). Available at https://easl.eu/wp-content/uploads/2019/07/Policy-statement-on-Food-obes....
-
- European Association for the Study of the Liver. ‘EASL clinical guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol.75, 659–689 (2021). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

