Thylakoid membrane remodeling by VIPP1 ESCRT-III-like filaments
- PMID: 40102625
- PMCID: PMC12081206
- DOI: 10.1038/s41594-025-01511-x
Thylakoid membrane remodeling by VIPP1 ESCRT-III-like filaments
Abstract
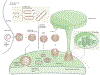
Three papers show that Vipp1, a plastid ESCRT-III protein, can form sheets, spirals and regular polygons on flat membranes and tubulate the membranes within stacked rings and helices. This work provides a framework for how Vipp1 can deliver lipids for thylakoid membrane biogenesis and protect and repair the membranes during photosynthesis.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
The cyanobacterial protein VIPP1 forms ESCRT-III-like structures on lipid bilayers.Nat Struct Mol Biol. 2025 Mar;32(3):543-554. doi: 10.1038/s41594-024-01367-7. Epub 2024 Jul 26. Nat Struct Mol Biol. 2025. PMID: 39060677 Free PMC article.
-
Mechanism for Vipp1 spiral formation, ring biogenesis, and membrane repair.Nat Struct Mol Biol. 2025 Mar;32(3):571-584. doi: 10.1038/s41594-024-01401-8. Epub 2024 Nov 11. Nat Struct Mol Biol. 2025. PMID: 39528797 Free PMC article.
-
Structural basis for VIPP1 oligomerization and maintenance of thylakoid membrane integrity.Cell. 2021 Jul 8;184(14):3643-3659.e23. doi: 10.1016/j.cell.2021.05.011. Epub 2021 Jun 23. Cell. 2021. PMID: 34166613
-
Principles of membrane remodeling by dynamic ESCRT-III polymers.Trends Cell Biol. 2021 Oct;31(10):856-868. doi: 10.1016/j.tcb.2021.04.005. Epub 2021 May 10. Trends Cell Biol. 2021. PMID: 33980463 Review.
-
Conserved structures of ESCRT-III superfamily members across domains of life.Trends Biochem Sci. 2023 Nov;48(11):993-1004. doi: 10.1016/j.tibs.2023.08.009. Epub 2023 Sep 15. Trends Biochem Sci. 2023. PMID: 37718229 Review.
References
-
- Schlosser L, Sachse C, Low HH & Schneider D Trends Biochem. Sci 48, 993–1004 (2023). - PubMed
-
- Blanch Jover A & Dekker C Trends Microbiol. 31, 601–615 (2023). - PubMed
-
- Zhang L & Sakamoto W Biochim. Biophys. Acta 1847, 831–837 (2015). - PubMed
-
- Pan S et al. Nat. Struct. Mol. Biol 10.1038/s41594-024-01367-7 (2024). - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

