Corticomuscular and intermuscular coherence during evidence accumulation in sensorimotor decision-making
- PMID: 40102698
- PMCID: PMC11919635
- DOI: 10.14814/phy2.70237
Corticomuscular and intermuscular coherence during evidence accumulation in sensorimotor decision-making
Abstract
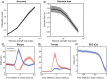
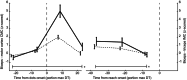
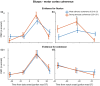
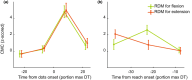
Evidence accumulation processes during decision-making are thought to continuously feed into the motor system, preparing multiple competing motor plans, of which one is executed when the evidence is complete. Previously, the state of this accumulation process has been studied by reading out the preparatory state of the motor system with evoked responses, once per trial. In this study, we aim to continuously track the sensorimotor decision during the trial using corticomuscular (CMC) and intermuscular coherence (IMC). We recorded EEG and EMG of healthy young adults (n = 34) who viewed random dot motion stimuli, with varying strengths across trials, and indicated their perceived motion direction by reaching towards one of two targets, requiring either flexion or extension of the elbow. Coherence was computed in the beta band. After stimulus presentation, both CMC and IMC show an initial phasic pattern, which is followed by sustained coherence patterns at a level that depends on stimulus strength for CMC. Prior to reach onset, the CMC for different stimulus strengths had a tendency to settle at similar levels. This tendency tentatively marks a stimulus-independent decision bound. We conclude that CMC, and to a lesser extent IMC, track the evidence accumulation process on a single trial.
Keywords: corticomuscular coherence; decision‐making; evidence accumulation; intermuscular coherence; motor control.
© 2025 The Author(s). Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Abnormal patterns of corticomuscular and intermuscular coherence in childhood dystonia.Clin Neurophysiol. 2020 Apr;131(4):967-977. doi: 10.1016/j.clinph.2020.01.012. Epub 2020 Feb 4. Clin Neurophysiol. 2020. PMID: 32067914 Free PMC article.
-
Brain-hemispheric differences in the premotor area for motor planning: An approach based on corticomuscular connectivity during motor decision-making.Neuroimage. 2025 May 15;312:121230. doi: 10.1016/j.neuroimage.2025.121230. Epub 2025 Apr 17. Neuroimage. 2025. PMID: 40252879 Free PMC article.
-
Functional connectivity in the neuromuscular system underlying bimanual coordination.J Neurophysiol. 2016 Dec 1;116(6):2576-2585. doi: 10.1152/jn.00460.2016. Epub 2016 Sep 14. J Neurophysiol. 2016. PMID: 27628205 Free PMC article.
-
Corticomuscular coherence behavior in fine motor control of force: a critical review.Rev Neurol. 2010 Nov 16;51(10):610-23. Rev Neurol. 2010. PMID: 21069640 Review. English, Spanish.
-
Corticomuscular coherence: a review.J Clin Neurophysiol. 1999 Nov;16(6):501-11. doi: 10.1097/00004691-199911000-00002. J Clin Neurophysiol. 1999. PMID: 10600018 Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

