Role of the oral-gut microbiota axis in pancreatic cancer: a new perspective on tumor pathophysiology, diagnosis, and treatment
- PMID: 40102723
- PMCID: PMC11917121
- DOI: 10.1186/s10020-025-01166-w
Role of the oral-gut microbiota axis in pancreatic cancer: a new perspective on tumor pathophysiology, diagnosis, and treatment
Abstract
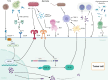
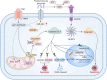
Pancreatic cancer, one of the most lethal malignancies, remains challenging due to late diagnosis, aggressive progression, and therapeutic resistance. Recent advances have revealed the presence of intratumoral microbiota, predominantly originating from the oral and gut microbiomes, which play pivotal roles in pancreatic cancer pathogenesis. The dynamic interplay between oral and gut microbial communities, termed the "oral-gut microbiota axis," contributes multifacetedly to pancreatic ductal adenocarcinoma (PDAC). Microbial translocation via anatomical or circulatory routes establishes tumor-resident microbiota, driving oncogenesis through metabolic reprogramming, immune regulation, inhibition of apoptosis, chronic inflammation, and dysregulation of the cell cycle. Additionally, intratumoral microbiota promote chemoresistance and immune evasion, further complicating treatment outcomes. Emerging evidence highlights microbial signatures in saliva and fecal samples as promising non-invasive diagnostic biomarkers, while microbial diversity correlates with prognosis. Therapeutic strategies targeting this axis-such as antibiotics, probiotics, and engineered bacteria-demonstrate potential to enhance treatment efficacy. By integrating mechanisms of microbial influence on tumor biology, drug resistance, and therapeutic applications, the oral-gut microbiota axis emerges as a critical regulator of PDAC, offering novel perspectives for early detection, prognostic assessment, and microbiome-based therapeutic interventions.
Keywords: Early diagnosis; Immune regulation; Oral-gut microbiota axis; Pancreatic cancer; Theraputic application.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Current status and prospect of gut and oral microbiome in pancreatic cancer: Clinical and translational perspectives.Cancer Lett. 2024 Nov 1;604:217274. doi: 10.1016/j.canlet.2024.217274. Epub 2024 Sep 21. Cancer Lett. 2024. PMID: 39307411 Review.
-
Upregulation of Lactobacillus spp. in gut microbiota as a novel mechanism for environmental eustress-induced anti-pancreatic cancer effects.Gut Microbes. 2025 Dec;17(1):2470372. doi: 10.1080/19490976.2025.2470372. Epub 2025 Feb 23. Gut Microbes. 2025. PMID: 39988618 Free PMC article.
-
Metagenomic Study Reveals Phage-Bacterial Interactome Dynamics in Gut and Oral Microbiota in Pancreatic Diseases.Int J Mol Sci. 2024 Oct 12;25(20):10988. doi: 10.3390/ijms252010988. Int J Mol Sci. 2024. PMID: 39456772 Free PMC article.
-
The potential of gut microbiome as a non-invasive predictive biomarker for early detection of pancreatic cancer and hepatocellular carcinoma.Eur Rev Med Pharmacol Sci. 2021 Dec;25(23):7275-7284. doi: 10.26355/eurrev_202112_27421. Eur Rev Med Pharmacol Sci. 2021. PMID: 34919227 Review.
-
Gut Microbiota: Its Potential Roles in Pancreatic Cancer.Front Cell Infect Microbiol. 2020 Oct 7;10:572492. doi: 10.3389/fcimb.2020.572492. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33117731 Free PMC article. Review.
Cited by
-
The Oral-Gut Microbiota Axis as a Mediator of Frailty and Sarcopenia.Nutrients. 2025 Jul 23;17(15):2408. doi: 10.3390/nu17152408. Nutrients. 2025. PMID: 40805993 Free PMC article. Review.
-
The Oral-Gut Microbiota Axis Across the Lifespan: New Insights on a Forgotten Interaction.Nutrients. 2025 Aug 1;17(15):2538. doi: 10.3390/nu17152538. Nutrients. 2025. PMID: 40806122 Free PMC article. Review.
-
Crosstalk between metabolic reprogramming and microbiota: implications for cancer progression and novel therapeutic opportunities.Front Immunol. 2025 May 20;16:1582166. doi: 10.3389/fimmu.2025.1582166. eCollection 2025. Front Immunol. 2025. PMID: 40463381 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

