Neurogenic differentiation 2 promotes inflammatory activation of macrophages in doxorubicin-induced myocarditis via regulating protein kinase D
- PMID: 40102732
- PMCID: PMC11916933
- DOI: 10.1186/s12872-025-04626-7
Neurogenic differentiation 2 promotes inflammatory activation of macrophages in doxorubicin-induced myocarditis via regulating protein kinase D
Abstract
Background: Although it has been established that protein kinase D (PKD) plays a crucial role in various diseases, its precise role in myocarditis remains elusive.
Methods: To investigate PKD's involvement in myocarditis, we established a mouse model of myocarditis using doxorubicin (DOX) to assess cardiac function, observe pathological changes, and quantify inflammatory cytokine levels in heart tissues. Additionally, macrophages were isolated from heart tissues of both control and DOX-treated groups to assess PKD expression and inflammatory cytokines in these macrophages. We explored the molecular mechanism of Neurogenic Differentiation 2 (NeuroD2) in myocarditis by utilizing NeuroD2 overexpression plasmids and NeuroD2 small interfering RNA (siRNA). Furthermore, we conducted dual-luciferase reporter and chromatin immunoprecipitation (ChIP) assays to investigate the interaction between NeuroD2 and PKD.
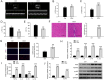
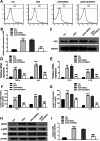
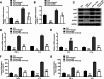
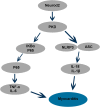
Results: We observed significant upregulation of PKD in macrophages and heart tissues induced by DOX. The administration of a PKD inhibitor reduced inflammatory cytokine levels, improved cardiac function, and mitigated pathological changes in myocarditis-afflicted mice. Mechanistically, we found upregulated expression of NeuroD2 in both macrophages and heart tissues exposed to DOX. NeuroD2 could directly target PKD, enhancing the NLRP3/NF-κB signaling pathway and exacerbating macrophage inflammation.
Conclusions: Our study demonstrates that NeuroD2 can directly bind to the PKD promoter, potentially promoting inflammatory activation of macrophages in DOX-induced myocarditis via the NLRP3/NF-κB pathway. This suggests that the NeuroD2/PKD axis may hold promise as a potential therapeutic approach for treating DOX-induced myocarditis.
Keywords: Inflammatory activation; Macrophages; Myocarditis; NLRP3/NF-κB pathway; NeuroD2; PKD.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All in vivo experiments were performed according to the guidelines for the Care and Use of Laboratory Animals of Xiangya Hospital of Central South University. This study was conducted in accordance with the ARRIVE Guidelines and approved by the Institutional Animal Care and Use Committee (IACUC) of Xiangya Hospital of Central South University (Approval No. 2022101065). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests. Clinical trial number: Not applicable.
Figures


References
-
- Ma WH, Zhang XG, Guo LL, Zhang JB, Wei FT, Lu QH, Du H, Kong YR, Wang X, Xu DL. Androgen receptor Inhibition alleviated inflammation in experimental autoimmune myocarditis by increasing autophagy in macrophages. Eur Rev Med Pharmacol Sci. 2021;25(10):3762–71. - PubMed
-
- Pollack A, Kontorovich AR, Fuster V, Dec GW. Viral myocarditis–diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015;12(11):670–80. - PubMed
-
- Kawada JI, Takeuchi S, Imai H, Okumura T, Horiba K, Suzuki T, Torii Y, Yasuda K, Imanaka-Yoshida K, Ito Y. Immune cell infiltration landscapes in pediatric acute myocarditis analyzed by CIBERSORT. J Cardiol. 2021;77(2):174–78. - PubMed
-
- Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R et al.,. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013; 34(33):2636-48, 48a-48d. - PubMed
-
- Li HD, Chen B, Yang F. IGF-1 affects the development of myocarditis in LDL-R knockout mice by inhibiting peritoneal infiltration of macrophages. Eur Rev Med Pharmacol Sci. 2020;24(15):8104–11. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

