Time course and determinants of the antibody response to SARS-CoV-2 in Costa Rica: the RESPIRA study
- PMID: 40102764
- PMCID: PMC11917050
- DOI: 10.1186/s12879-025-10742-8
Time course and determinants of the antibody response to SARS-CoV-2 in Costa Rica: the RESPIRA study
Abstract
Background: Antibodies to SARS-CoV-2 are essential for protection or reduction in severity of subsequent disease. We studied antibody responses to spike protein receptor-binding domain (S1-RBD) and nucleocapsid (N) in a population-based sample of COVID-19 cases in Costa Rica.
Methods: As part of the RESPIRA study, we selected an age-stratified random sample of PCR-confirmed COVID-19 cases diagnosed from March 2020 to July 2021. Antibodies were determined with multiplex serology in 794 unvaccinated subjects diagnosed 3 days to 17 months before recruitment to investigate immune response to natural infection. In addition, neutralizing antibodies were determined in 136 randomly selected participants. We estimated antibody positivity and GMTs by time since diagnosis and explored determinants using multivariate regression.
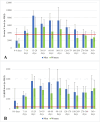
Results: Most participants tested 15-29 days after PCR diagnosis were seropositive for N (90%) and S1-RBD antibodies (96%) and had the highest GMTs for both antibodies. Only 42% of subjects tested one year after infection were seropositive for N antibodies, compared to 97% for S1-RBD. GMTs for neutralizing antibodies peaked 15-89 days after infection and declined but remained positive for 95% of subjects thereafter. In multivariate models, antibodies were significantly higher among men and increased with age and severity of the clinical presentation. The correlation of multiplex and neutralizing antibodies was high (0.72 [95% CI = 0.63-0.79]) and stronger among women.
Conclusions: A robust immune response against N and S1-RBD is elicited by COVID-19 a few days after infection. While S1-RBD antibodies are present after > 1 year, N antibodies decline significantly. Antibody levels are higher in men and increase with age and severity of disease. The different immune response patterns by sex warrant further investigation.
Trial registration: RESPIRA Study ClinicalTrials.gov ID: NCT04537338 (3 September 2020).
Keywords: Antibody response; COVID-19; Costa Rica; RESPIRA; SARS-CoV-2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The RESPIRA study was approved by the Ethical Committee of the Costa Rican Social Security Administration (Caja Costarricense de Seguro Social), protocol #R020-SABI-00261. The committee is authorized and supervised by the National Research Council (CONIS) of the Ministry of Health of Costa Rica under Research Law number 9234 of April 2014. The research was conducted in compliance with all local regulations and the Declaration of Helsinki. All participants, or their parents in the case of minors, signed informed consent forms approved by the ethical committee. Children between 12 and 17 additionally signed informed assent forms. The signature was performed in the presence of a witness who was not part of the study staff, according to the Costa Rican research law. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- World Health Organization. WHO Coronavirus (COVID-19) dashboard 2024 [Available from: https://data.who.int/dashboards/covid19/cases.
-
- United Nations. WHO chief declares end to COVID-19 as a global health emergency 2023 [Available from: https://news.un.org/en/story/2023/05/1136367.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

