The influence of extrinsic apoptosis gene expression on immunological reconstitution of male ART-treated PLHIV
- PMID: 40102787
- PMCID: PMC11921504
- DOI: 10.1186/s12879-025-10665-4
The influence of extrinsic apoptosis gene expression on immunological reconstitution of male ART-treated PLHIV
Abstract
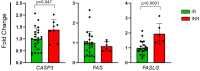
The primary goal of antiretroviral therapy (ART) is to suppress viral replication to undetectable levels (< 50 copies/mL). Despite achieving complete viral suppression, 10-40% of individuals on ART do not adequately restore their CD4 + T-cell count, being defined as immunological non-responders (INR). Factors such as sex, age at treatment initiation, coinfections, and pre-ART CD4 + T-cell count may influence this insufficient recovery. This impairment can also result from poor production or exacerbated destruction of CD4 + T-cells, particularly through extrinsic pathway-mediated apoptosis involving Fas/FasL and caspase-3. Thus, this study aimed to evaluate the expression profile of extrinsic apoptosis pathway genes (CASP3, FAS, FASLG) in adult male HIV patients on ART. The patients were stratified as immunological responders (n = 25) and immunological non-responders (n = 8) based on the increase and total count of CD4 + T-cells. Significant differences for CASP3 (FC = 1.39, p = 0.047) and FASLG (FC = 1.94, p < 0.0001) gene expressions were identified between IR and INR groups, but not for FAS (FC=-1.2, p = 0.638). This study indicates increased apoptotic pathway gene expression in INR and highlights the influence of cell destruction mechanisms on immunological recovery.
Keywords: CASP3; CD4 + T-cell recovery; FAS; FASL; cell death; immunological non-responders.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Instituto de Medicina Integral Professor Fernando Figueira (protocol code: 3629-13; 13 November 2013). Informed consent was obtained from all subjects involved in this study. Consent for publication: Not applicable. Clinical trial: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

