Tofacitinib for ulcerative colitis in Brazil: a multicenter observational study on effectiveness and safety
- PMID: 40102788
- PMCID: PMC11921721
- DOI: 10.1186/s12876-025-03656-x
Tofacitinib for ulcerative colitis in Brazil: a multicenter observational study on effectiveness and safety
Abstract
Aim: To assess the real-life, long-term effectiveness and safety of tofacitinib in a large cohort of patients with refractory or difficult-to-treat ulcerative colitis (UC).
Methods: This multicenter, retrospective, observational cohort study included patients with moderately to severely active UC who received tofacitinib for at least 8 weeks. Clinical remission and response, endoscopic response and remission, biochemical response and remission, steroid-free clinical remission, primary and secondary loss of response, drug discontinuation, the need for dose optimization, the need for colectomy, and adverse events were evaluated over up to 30 months.
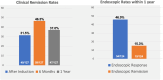
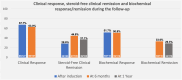
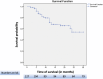
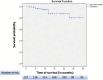
Results: We included 127 patients with UC, with a mean age of 40.3 ± 14.2 years; 58.2% were male, 75.6% had pancolitis, and 79.5% had previously failed at least one biological therapy, predominantly anti-TNF agents (70.1%). Clinical remission was observed in 31.5% of patients at weeks 12-16, 46.5% at 26 ± 4 weeks, and 37.0% at 1 year. Steroid-free clinical remission was achieved in 28.6%, 44.8%, and 37.1% of patients at the same time points, respectively. Biochemical remission was achieved in 33.6% of patients at 26 ± 4 weeks and 29.3% at 1 year. Endoscopic response and endoscopic remission within 1 year were observed in 46.0% and 15.3% of patients, respectively. Ten patients (7.9%) required colectomy, and 13 patients (10.2%) required hospitalization, all of whom had been previously exposed to biologics. The colectomy rate was significantly greater in patients with serum albumin levels ≤ 3.5 g/dL (21.4% vs. 4.1%, p = 0.013).
Conclusion: In this large, long-term real-world study involving patients with predominantly biologically refractory UC, tofacitinib effectively induced clinical remission and endoscopic improvement and prevented colectomy for more than 30 months, with a favorable safety profile. Notably, baseline hypoalbuminemia was associated with higher colectomy rates.
Keywords: Inflammatory bowel disease; Jak inhibitors; Tofacitinib; Real-world; Ulcerative colitis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Ethics Committee of the coordinating site, the Hospital das Clínicas of the Ribeirão Preto Medical School at the University of São Paulo, with the following approval details: CAAE 71008923.0.1001.5440; Ethics Committee Number 6.171.795/2023. Based on this ethical approval, we are authorized to retrospectively collect data from all patients receiving treatment with small molecules, such as tofacitinib. All procedures were conducted in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Consent for publication: Not Applicable. Competing interests: Parra RS has received fees for serving as a speaker and/or an advisory board member for Takeda, Janssen, Abbvie, Celltrion and Pfizer. Fróes RSB has received fees for serving as a speaker and/or an advisory board member for Takeda, Janssen, Abbvie, and is a speaker for Celltrion, Nestle, Ferring, Pfizer, and CSL Vifor. Ferreira SC has received fees for serving as a speaker and/or an advisory board member for Janssen, Takeda, and Pfizer. Mello MK has received fees for serving as a speaker and/or an advisory board member for Takeda, Janssen, Abbvie, Sandoz and Ferring. Azevedo MFC has received fees for serving as a speaker and/or an advisory board member for Takeda, Abbvie, Pfizer and Janssen. Damião AOMC has received fees for serving as a speaker and/or an advisory board member for Takeda, Abbvie, Ferring, and Janssen Carlos AS has received fees for serving as a speaker and/or an advisory board member for Takeda, Janssen, Abbvie Miranda MLQ has received fees for serving as speaker for Takeda, Janssen and Abbvie Zabot GP has received fees for serving as a speaker and/or an advisory board member for Janssen, Abbvie, and Takeda. Cassol OS has received fees for serving as a speaker and/or an advisory board member for Nestle, Abbvie, Janssen, Takeda, and Buhlmann. Alves Junior AJT has received fees for serving as a speaker and/or an advisory board member for Janssen, Takeda, UCB, and Abbvie. Machado MB has received fees for serving as a speaker and/or na Advisory board for Takeda, Jassen, Abbvie, Ferring and Pfizer. Teixeira FV is a speaker and an advisory board member of Takeda. Chebli JMF has received fees for serving as a speaker and/or an advisory board member for Takeda, Janssen, AbbVie, Abbott, and Sandoz. Magro, DO, Sales MPM, Barros LL, Sassaki LY, Flores C, Vieira A, Lubini M, Coy CSR, Zaltmann C, and Féres O report no conflicts of interest.
Figures
Similar articles
-
Effect of Tofacitinib on One-Year Colectomy Risk in Anti-TNF Refractory Ulcerative Colitis: A Prospective Multicenter Italian Study.Dig Dis Sci. 2024 May;69(5):1785-1792. doi: 10.1007/s10620-024-08394-w. Epub 2024 Mar 26. Dig Dis Sci. 2024. PMID: 38530500
-
Real-world evidence of tofacitinib effectiveness and safety in patients with refractory ulcerative colitis.Dig Liver Dis. 2020 Mar;52(3):268-273. doi: 10.1016/j.dld.2019.10.003. Epub 2019 Nov 13. Dig Liver Dis. 2020. PMID: 31732444
-
Short-term effectiveness and safety of tofacitinib in ulcerative colitis - real world data from tertiary medical centers in Israel.Dig Liver Dis. 2022 Feb;54(2):192-197. doi: 10.1016/j.dld.2021.11.009. Epub 2021 Dec 7. Dig Liver Dis. 2022. PMID: 34887214
-
Oral Janus kinase inhibitors for maintenance of remission in ulcerative colitis.Cochrane Database Syst Rev. 2020 Jan 27;1(1):CD012381. doi: 10.1002/14651858.CD012381.pub2. Cochrane Database Syst Rev. 2020. PMID: 31984480 Free PMC article.
-
Real-World Effectiveness and Safety of Tofacitinib in Patients With Ulcerative Colitis: Systematic Review With Meta-Analysis.Inflamm Bowel Dis. 2022 Jan 5;28(1):32-40. doi: 10.1093/ibd/izab011. Inflamm Bowel Dis. 2022. PMID: 33586766
References
-
- Gros B, Kaplan GG. Ulcerative Colitis in Adults: A Review. JAMA. 2023;330(10):951–65. 10.1001/jama.2023.15389. - PubMed
-
- Neurath MF, Vieth M. Different levels of healing in inflammatory bowel diseases: mucosal, histological, transmural, barrier and complete healing. Gut. 2023;72(11):2164–83. 10.1136/gutjnl-2023-329964. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

