Establishment of CD34 + hematopoietic stem cell-derived xenograft model of hyperleukocytic acute myeloid leukemia
- PMID: 40102796
- PMCID: PMC11917077
- DOI: 10.1186/s12885-025-13907-5
Establishment of CD34 + hematopoietic stem cell-derived xenograft model of hyperleukocytic acute myeloid leukemia
Abstract
Background: Hyperleukocytic acute myeloid leukemia (HLL) is marked by high early mortality and presents significant therapeutic challenges. Research on HLL is still in its infancy, and comprehensive development of patient-derived xenograft (PDX) models, especially CD34 + hematopoietic stem cell-derived models, remains limited.
Methods: We evaluated the establishment of the HLL model through blood examinations, smear analysis, bone marrow biopsy, flow cytometry, and mutation analysis. Correlation between survival times in mice and patients was assessed using linear regression.
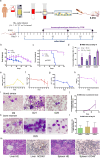
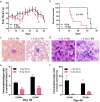
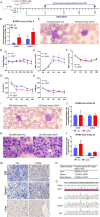
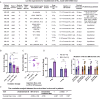
Results: In the HLL PDX mouse model, leukocyte counts could reach up to 37.35^10⁹/L, and immunophenotyping revealed the presence of hCD45+, hCD15+, and hCD33 + cells in both peripheral blood (PB) and bone marrow (BM) following inoculation with PB-derived cells for the establishment of the HLL PDX model. Similar results were observed with cells derived from the patient's BM. In the CD34 + hematopoietic stem cell-derived xenograft model, extensive infiltration of CD34 + cells into the BM, liver, and spleen was observed. Additionally, human WT1 and NRAS mutations were identified in the liver, spleen, and BM of the mice. A comparative analysis of multiple experiments revealed that shorter survival times were observed in mice receiving a higher irradiation dose of 2.5 Gy and a greater number of cells derived from PB. Additionally, shorter survival times were observed in model mice injected with cells carrying NRAS, DNMT3A, FLT3, or NPM1 gene mutations. Correlation analysis indicated that the survival times of the mice were significantly associated with the survival status of the patients.
Conclusions: We successfully established a CD34 + hematopoietic stem cell-derived xenograft model of HLL, providing a valuable tool for mechanistic research, drug screening, individualized therapy, and precision medicine.
Trial registration: Not application.
Keywords: B-NSG mice; CD34 + hematopoietic stem cell; Hyperleukocytic acute myeloid leukemia; Patient-derived xenograft model.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was carried out followed the Declaration of Helsinki and approved by the Research Ethics Committee of Zhongnan Hospital at Wuhan University (license number: 2017048). Informed written consent was obtained from the HLL patients. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Feasibility Assessment of Autologous Human Immune System (HIS) ImmunoGraft Platform Development Using Autologous Mobilized Peripheral Blood (MPB) CD34 Cells Derived from Adult HNSCC Patient.Int J Mol Sci. 2025 May 30;26(11):5269. doi: 10.3390/ijms26115269. Int J Mol Sci. 2025. PMID: 40508078 Free PMC article.
-
RHAMM/HMMR (CD168) is not an ideal target antigen for immunotherapy of acute myeloid leukemia.Haematologica. 2012 Oct;97(10):1539-47. doi: 10.3324/haematol.2012.065581. Epub 2012 Apr 24. Haematologica. 2012. PMID: 22532518 Free PMC article.
-
CD34+ cells from AML with mutated NPM1 harbor cytoplasmic mutated nucleophosmin and generate leukemia in immunocompromised mice.Blood. 2010 Nov 11;116(19):3907-22. doi: 10.1182/blood-2009-08-238899. Epub 2010 Jul 15. Blood. 2010. PMID: 20634376
-
Immunophenotype and functional characteristics of human primitive CD34-negative hematopoietic stem cells: the significance of the intra-bone marrow injection.J Autoimmun. 2008 May;30(3):136-44. doi: 10.1016/j.jaut.2007.12.004. J Autoimmun. 2008. PMID: 18243660 Review.
-
Advances in the application of patient-derived xenograft models in acute leukemia resistance.Cancer Drug Resist. 2025 May 28;8:23. doi: 10.20517/cdr.2025.18. eCollection 2025. Cancer Drug Resist. 2025. PMID: 40510031 Free PMC article. Review.
References
-
- Giammarco S, Chiusolo P, Piccirillo N, Di Giovanni A, Metafuni E, Laurenti L, et al. Hyperleukocytosis and leukostasis: management of a medical emergency. Expert Rev Hematol. 2017;10(2):147–54. - PubMed
-
- Feng S, Zhou L, Zhang X, Tang B, Zhu X, Liu H, et al. Impact Of ELN Risk Stratification, Induction Chemotherapy Regimens And Hematopoietic Stem Cell Transplantation On Outcomes In Hyperleukocytic Acute Myeloid Leukemia With Initial White Blood Cell Count More Than 100 x 10(9)/L. Cancer Manag Res. 2019;11:9495–503. - PMC - PubMed
-
- Rollig C, Ehninger G. How I treat hyperleukocytosis in acute myeloid leukemia. Blood. 2015;125(21):3246–52. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

