Ubiquitin-specific protease: an emerging key player in cardiomyopathy
- PMID: 40102846
- PMCID: PMC11921692
- DOI: 10.1186/s12964-025-02123-0
Ubiquitin-specific protease: an emerging key player in cardiomyopathy
Abstract
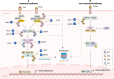
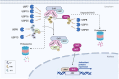
Protein quality control (PQC) plays a vital role in maintaining normal heart function, as cardiomyocytes are relatively sensitive to misfolded or damaged proteins, which tend to accumulate under pathological conditions. Ubiquitin-specific protease (USP) is the largest deubiquitinating enzyme family and a key component of the ubiquitin proteasome system (UPS), which is a non-lysosomal protein degradation machinery to mediate PQC in cells. USPs regulate the stability or activity of the target proteins that involve intracellular signaling, transcriptional control of inflammation, antioxidation, and cell growth. Recent studies demonstrate that the USPs can regulate fibrosis, lipid metabolism, glucose homeostasis, hypertrophic response, post-ischemic recovery and cell death such as apoptosis and ferroptosis in cardiomyocytes. Since myocardial cell loss is an important component of cardiomyopathy, therefore, these findings suggest that the UPSs play emerging roles in cardiomyopathy. This review briefly summarizes recent literature on the regulatory roles of USPs in the occurrence and development of cardiomyopathy, giving us new insights into the molecular mechanisms of USPs in different cardiomyopathy and potential preventive strategies for cardiomyopathy.
Keywords: Cardiomyopathy; Deubiquitination; PQC; USP.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
The ubiquitin-proteasome system: focus on the heart.Cardiovasc Res. 2006 Jun 1;70(3):410-21. doi: 10.1016/j.cardiores.2005.12.021. Epub 2006 Feb 23. Cardiovasc Res. 2006. PMID: 16497285 Review.
-
Targeting ubiquitin specific proteases (USPs) in cancer immunotherapy: from basic research to preclinical application.J Exp Clin Cancer Res. 2023 Sep 1;42(1):225. doi: 10.1186/s13046-023-02805-y. J Exp Clin Cancer Res. 2023. PMID: 37658402 Free PMC article. Review.
-
Role of the ubiquitin-proteasome system in brain ischemia: friend or foe?Prog Neurobiol. 2014 Jan;112:50-69. doi: 10.1016/j.pneurobio.2013.10.003. Epub 2013 Oct 22. Prog Neurobiol. 2014. PMID: 24157661 Review.
-
The deubiquitinase USP5 prevents accumulation of protein aggregates in cardiomyocytes.Sci Adv. 2025 Jan 24;11(4):eado3852. doi: 10.1126/sciadv.ado3852. Epub 2025 Jan 22. Sci Adv. 2025. PMID: 39841822 Free PMC article.
-
The ubiquitin-proteasome system in cardiac proteinopathy: a quality control perspective.Cardiovasc Res. 2010 Jan 15;85(2):253-62. doi: 10.1093/cvr/cvp287. Epub 2009 Aug 20. Cardiovasc Res. 2010. PMID: 19696071 Free PMC article. Review.
Cited by
-
Molecular Insights into Oxidative-Stress-Mediated Cardiomyopathy and Potential Therapeutic Strategies.Biomolecules. 2025 May 6;15(5):670. doi: 10.3390/biom15050670. Biomolecules. 2025. PMID: 40427563 Free PMC article. Review.
References
-
- Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. 2006;113:1807–16. - PubMed
-
- Dantuma NP, Lindsten K. Stressing the ubiquitin-proteasome system. Cardiovasc Res. 2010;85:263–71. 10.1093/cvr/cvp255. - PubMed
-
- Han D, Wang L, Jiang S, Yang Q. The ubiquitin-proteasome system in breast cancer. Trends Mol Med. 2023;29:599–621. 10.1016/j.molmed.2023.05.006. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

