Cellular sentinels: empowering survival and immune defense in hematopoietic stem cell transplantation through mesenchymal stem cells and T lymphocytes
- PMID: 40102849
- PMCID: PMC11921582
- DOI: 10.1186/s12916-025-03987-2
Cellular sentinels: empowering survival and immune defense in hematopoietic stem cell transplantation through mesenchymal stem cells and T lymphocytes
Abstract
Background: Hematopoietic stem cell transplantation (HSCT) is a critical treatment for hematologic disorders such as leukemia, lymphoma, and specific immune deficiencies. Despite its efficacy, challenges such as engraftment failure and delayed neutrophil regeneration remain significant barriers. These complications lead to prolonged cytopenia, increased risks of infections and other complications, and elevated morbidity and mortality rates. While mesenchymal stem cells (MSCs) are known to play essential roles in supporting hematopoiesis, the precise mechanisms and interactions between MSCs and other cellular components in HSCT require further investigation.
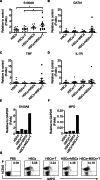
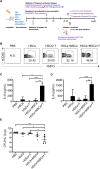
Methods: To address these challenges, we explored the combined infusion of allotype-cord blood hematopoietic stem cells (HSCs) and activated T cells from the same donor along with third-party MSCs. The study assessed the effects of this triple-cell therapy on neutrophil differentiation and function ex vivo and in vivo. Using a respiratory infection model, we evaluated the accumulation of human neutrophils, cytokine secretion (IL-6 and IL-8), bacterial clearance, and overall survival compared to control groups.
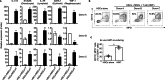
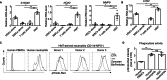
Results: The triple-cell therapy demonstrated a significant improvement in the differentiation of human HSCs into neutrophils both in ex vivo and in vivo. In the respiratory infection model, this approach resulted in enhanced accumulation of human neutrophils, increased secretion of IL-6 and IL-8, superior bacterial clearance, and reduced mortality rates compared to the control group. These findings highlight the synergistic interplay between allo-HSCs, MSCs, and activated T cells in promoting neutrophil production and function.
Conclusions: Our study presents a novel therapeutic strategy combining allo-HSCs, activated T cells, and third-party MSCs to enhance neutrophil production and functionality post-transplantation. This approach not only accelerates neutrophil regeneration but also improves resistance to infections, offering a promising avenue to overcome engraftment challenges in HSCT.
Keywords: Activated T cells; Engraftment enhancement; Hematopoietic stem cell transplantation (HSCT); Mesenchymal stem cells (MSCs); Neutrophil regeneration.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was performed in accordance with the Declaration of Helsinki and approved by the institutional review board of Taoyuan General Hospital, Ministry of Health and Welfare, Taiwan (TYGH100040, TYGH109026), and the Research Ethics Committee of National Health Research Institutes (EC1000802, EC1001101-R1, EC1030604-E, EC1111003-W, EC1000802, EC1030604-E, EC1111215-W, and EC1100308-E). All written informed consents were obtained from participants of this study. Consent for publication: All authors consent to publication. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Mesenchymal stem cell infusion for enhancing hematopoietic recovery and addressing cytopenias in CAR-T cell therapy.Stem Cell Res Ther. 2024 Sep 27;15(1):333. doi: 10.1186/s13287-024-03941-8. Stem Cell Res Ther. 2024. PMID: 39334276 Free PMC article.
-
Dual-Double Stem Cell Ovarian Therapy: A Comprehensive Approach in Regenerative Medicine.Int J Mol Sci. 2024 Dec 25;26(1):69. doi: 10.3390/ijms26010069. Int J Mol Sci. 2024. PMID: 39795929 Free PMC article. Review.
-
Chimerism of bone marrow mesenchymal stem/stromal cells in allogeneic hematopoietic cell transplantation: is it clinically relevant?Chimerism. 2013 Jul-Sep;4(3):78-83. doi: 10.4161/chim.25609. Epub 2013 Jul 11. Chimerism. 2013. PMID: 23880502 Free PMC article. Review.
-
The role of mesenchymal stem cells in hematopoietic stem cell transplantation: prevention and treatment of graft-versus-host disease.Stem Cell Res Ther. 2019 Jun 21;10(1):182. doi: 10.1186/s13287-019-1287-9. Stem Cell Res Ther. 2019. PMID: 31227011 Free PMC article. Review.
-
T Cell and Cytokine Dynamics in the Blood of Patients after Hematopoietic Stem Cell Transplantation and Multipotent Mesenchymal Stromal Cell Administration.Transplant Cell Ther. 2023 Feb;29(2):109.e1-109.e10. doi: 10.1016/j.jtct.2022.10.030. Epub 2022 Nov 11. Transplant Cell Ther. 2023. PMID: 36372356 Clinical Trial.
References
-
- Crane GM, Jeffery E, Morrison SJ. Adult haematopoietic stem cell niches. Nat Rev Immunol. 2017;17(9):573–90. - PubMed
-
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813–26. - PubMed
-
- Adams GB, Martin RP, Alley IR, Chabner KT, Cohen KS, Calvi LM, Kronenberg HM, Scadden DT. Therapeutic targeting of a stem cell niche. Nat Biotechnol. 2007;25(2):238–43. - PubMed
-
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–6. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

