Increasing day three parasitaemia is observed after treatment of patients with artemether-lumefantrine and single dose of primaquine for uncomplicated Plasmodium falciparum malaria in Arbaminch Zuria district, Southwest Ethiopia
- PMID: 40102850
- PMCID: PMC11921638
- DOI: 10.1186/s12936-025-05337-2
Increasing day three parasitaemia is observed after treatment of patients with artemether-lumefantrine and single dose of primaquine for uncomplicated Plasmodium falciparum malaria in Arbaminch Zuria district, Southwest Ethiopia
Abstract
Background: Since 2017, the first-line treatment for uncomplicated Plasmodium falciparum infection in Ethiopia has been artemether-lumefantrine (AL), plus a single dose of primaquine (PQ). The World Health Organization (WHO) recommends regular monitoring of the first-line drug efficacy as crucial tool for supporting national treatment policies. This study aimed to assess the efficacy of AL with single dose of PQ for the treatment of uncomplicated P. falciparum malaria.
Methods: A prospective single-arm efficacy study was conducted among outpatients at Shecha Health Centre, aged six months and older with confirmed uncomplicated P. falciparum malaria from October 2023 to January 2024. Participants were treated with AL plus single dose of PQ and followed up to 28 days to evaluate clinical and parasitological responses. Kaplan-Meier (KM) survival analysis and per protocol (PP) analysis were used to estimate primary and secondary outcomes. Paired sample t-test was used to compare mean haemoglobin levels across follow-up dates (SPSS v.25). All comparisons were made at 95% confidence interval (CI), with a level of significance at 0.05.
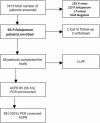
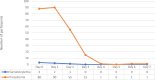
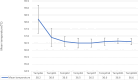
Results: A total of 93 patients with uncomplicated P. falciparum were enrolled and 88 participants completed the study. Based on KM analysis the overall PCR uncorrected cure rate of AL plus single dose of PQ was 96.6% (95% CI 90.4-99.3%). The PCR-corrected cure rate was 100% (95% CI 95.8-100%). Despite high cure rate, accompanied by fast resolution of clinical symptoms, 17% of participants continued to have detectable parasitaemia on day 3. There was a slight decrease in mean haemoglobin on day 28 compared to the baseline. No serious adverse events were reported during the 28-day follow-up period.
Conclusion: The study findings reaffirm high efficacy of AL with a single dose of PQ for the treatment of uncomplicated P. falciparum malaria, with acceptable safety profile. These findings support the ongoing use of AL with a single dose of PQ as the primary treatment option for uncomplicated P. falciparum infections in the study area. In the context of the emergence and spread of partial artemisinin-based combination therapy(ACT) resistance in Africa, regular monitoring of the efficacy of current artemisinin-based combinations is recommended for the early detection of emerging drug resistance in the study setting and beyond.
Keywords: Plasmodium-falciparum; Artemether-lumefantrine; Efficacy; Ethiopia; Safety.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approval was obtained from the Scientific and Ethics Review Committee of the Centre for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa), College of Health Sciences, Addis Ababa University (Protocol number:1851/23) and the Ethiopian Public Health Institute (EPHI) (Protocol number:294/2022) IRB office prior to the study’s initiation. Permission was obtained from Shecha Health Centre. Written informed consent was obtained from each adult patient and from parent or guardian for children. Additionally, assent was obtained from children aged 12–17 years. Study details, including potential benefits and potential risks, were thoroughly explained to study participants before enrollment. Confidentiality was maintained, and participants were informed of their right to withdraw from the study at any time. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Adding a single low-dose of primaquine (0.25 mg/kg) to artemether-lumefantrine did not compromise treatment outcome of uncomplicated Plasmodium falciparum malaria in Tanzania: a randomized, single-blinded clinical trial.Malar J. 2016 Aug 26;15(1):435. doi: 10.1186/s12936-016-1430-3. Malar J. 2016. PMID: 27565897 Free PMC article. Clinical Trial.
-
Therapeutic efficacy and safety of artemether-lumefantrine combination therapy for the treatment of uncomplicated Plasmodium falciparum malaria at Teda Health Centre, Northwest Ethiopia, 2022/23.Malar J. 2024 Aug 30;23(1):266. doi: 10.1186/s12936-024-05082-y. Malar J. 2024. PMID: 39215366 Free PMC article.
-
Parasite clearance, cure rate, post-treatment prophylaxis and safety of standard 3-day versus an extended 6-day treatment of artemether-lumefantrine and a single low-dose primaquine for uncomplicated Plasmodium falciparum malaria in Bagamoyo district, Tanzania: a randomized controlled trial.Malar J. 2020 Jun 23;19(1):216. doi: 10.1186/s12936-020-03287-5. Malar J. 2020. PMID: 32576258 Free PMC article. Clinical Trial.
-
Efficacy of dihydroartemisinin-piperaquine versus artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria among children in Africa: a systematic review and meta-analysis of randomized control trials.Malar J. 2021 Aug 12;20(1):340. doi: 10.1186/s12936-021-03873-1. Malar J. 2021. PMID: 34384431 Free PMC article.
-
Efficacy and safety of dihydroartemisinin-piperaquine versus artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Ugandan children: a systematic review and meta-analysis of randomized control trials.Malar J. 2021 Apr 1;20(1):174. doi: 10.1186/s12936-021-03711-4. Malar J. 2021. PMID: 33794897 Free PMC article.
References
-
- WHO. Guidelines for malaria 2023 [Internet]. Geneva, World Health Organization, 2023. Available from: https://www.who.int/publications/i/item/guidelines-for-malaria.
-
- WHO. World malaria report 2024. Geneva, World Health Organization, 2024. https://www.who.int/teams/global-malaria-programme/reports/world-malaria...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous