Development of an efficient expression system for human chaperone BiP in Pichia pastoris: production optimization and functional validation
- PMID: 40102863
- PMCID: PMC11917157
- DOI: 10.1186/s12934-025-02679-z
Development of an efficient expression system for human chaperone BiP in Pichia pastoris: production optimization and functional validation
Abstract
Background: Human BiP, or GRP78, is a molecular chaperone mainly found in the endoplasmic reticulum (ER). However, a growing amount of data also associates BiP with many distinct functions in subcellular locations outside the ER. Notably, several diseases have been BiP-related, so the protein could potentially be used for therapeutic purposes. This study aimed to optimize a high cell-density fermentation process for the production of recombinant human BiP (rhBiP) in yeast Pichia pastoris in a mineral medium.
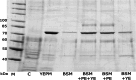
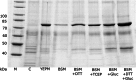
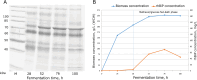
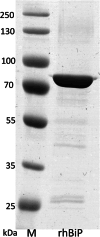
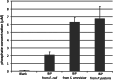
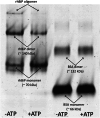
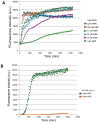
Results: P. pastoris cells successfully synthesized and secreted full-length rhBiP protein in a complex growth medium. However, secreted rhBiP titer was considerably lower when P. pastoris was cultivated in a defined mineral basal salt medium (BSM). During rhBiP synthesis optimization in shake flasks, it was found that the addition of reducing compounds (DTT or TCEP) to mineral BSM medium is essential for high-yield rhBiP production. Furthermore, rhBiP secretion in the BSM medium was significantly increased by feeding yeast with an additional carbon source. The addition of 2 mM DTT and 0.5-1.0% of glucose/glycerol to the BSM medium increased rhBiP titer ~ 8 times in the shake flasks. Glucose/methanol mixture feeding with added 2 mM DTT before induction was applied in high-density P. pastoris fermentation in bioreactor. Oxygen-limited fermentation strategy allowed to achieve ~ 70 mg/L rhBiP in BSM medium. Hydrophobic interaction and anion exchange chromatography were used for rhBiP protein purification. Approximately 45 mg rhBiP was purified from 1 L growth medium, and according to SDS-PAGE, ~ 90% purity was reached. According to data presented in this study, rhBiP protein derived from P. pastoris is a full-length polypeptide that has ATPase activity. In addition, we show that P. pastoris-derived rhBiP effectively inhibits neurodegenerative disease-related amyloid beta 1-42 (Aβ42) peptide and alpha-synuclein (α-Syn) protein aggregation in vitro.
Conclusions: A scalable bioprocess to produce rhBiP in P. pastoris was developed, providing a high yield of biologically active protein in a chemically defined mineral medium. It opens a source of rhBiP to accelerate further therapeutic applications of this important protein.
Keywords: Pichia pastoris; BiP; DTT; Fermentation; Mineral medium; Mixed feeding; Secretion.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: A part of the work presented in this paper was filed as international patent application.
Figures

References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

