Shengui Sansheng San alleviates the worsening of blood-brain barrier integrity resulted from delayed tPA administration through VIP/VIPR1 pathway
- PMID: 40102879
- PMCID: PMC11916937
- DOI: 10.1186/s13020-025-01079-0
Shengui Sansheng San alleviates the worsening of blood-brain barrier integrity resulted from delayed tPA administration through VIP/VIPR1 pathway
Abstract
Background: Intravenous tissue plasminogen activator (tPA) is currently the only FDA-approved thrombolytic therapy for acute ischemic stroke (AIS), however, relative narrow therapeutic time window (within 4.5 h of AIS onset) and high risk of hemorrhagic transformation due to blood-brain barrier (BBB) disruption limit tPA therapeutic benefits for patients. In this study, we extended the time window of tPA administration (5 h after the occurrence of AIS) and investigated whether Chinese medicine classical formula Shengui Sansheng San (SSS) administration was able to alleviate BBB integrity worsening, and the mechanism was related to vasoactive intestinal peptide (VIP)/ VIP receptor 1 (VIPR1) pathway.
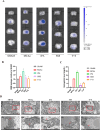
Methods: SSS was extracted using aqueous heating method and SFE-CO2 technology, and quality control was performed using UHPLC/MS analysis. Male C57BL/6 mice were suffered from middle cerebral artery occlusion (MCAo), followed by the removal of a silicone filament after 5 h, then, t-PA was administered via tail vein injection at once, along with SSS administration by gavage. Hemoglobin levels and Evans blue leakage were measured to assess brain hemorrhagic transformation and BBB permeability, respectively. Transmission electron microscope (TEM) was utilized to present brain microvascular endothelial cells (BMECs) tight junction morphology. TTC staining and laser speckle contrast imaging were employed for infarct volume and cerebral blood flow measurements. The modified neurological severity score (mNSS) test was conducted to evaluate neurological function. The expressions of VIP, VIPR1, ZO-1, Occludin, Lectin, GFAP, NeuN were detected by immunofluorescence staining or western blotting. In vitro, bEnd.3 and N2a cells were insulted by oxygen-glucose deprivation (OGD), and VIPR1 siRNA, and VIP shRNA transfection were respectively performed, and the molecular docking was applied to verify the SSS in-serum active compounds interacted with VIPR1. The transwell system was utilized to detect OGD-insulted BMECs permeability.
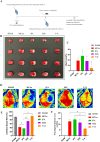
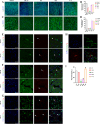
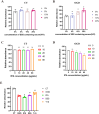
Results: SSS treatment significantly reduced the infarct area, cerebral hemorrhage, and neurological deficits, and enhanced cerebral blood flow in AIS mice received intravenous tPA beyond 4.5 h time window. Simultaneously, the permeability of BBB declined, with increased expressions of tight junction proteins ZO-1, and Occludin and proper BMECs tight junction morphology, and it suggested that VIP was released by neurons rather than astrocytes or BMECs. It also showed high expressions of VIP and VIPR1 in the penumbra area. The inhibition of VIP in N2a cells or VIPR1 in bEnd.3 cells abolished the viability and integrity of OGD-insulted bEnd.3 cells treated by tPA after SSS-containing serum administration, and the SSS in-serum active compounds were proved have high affinity to VIPR1 by molecular docking.
Conclusion: SSS alleviates the worsening of BBB integrity resulted from delayed tPA administration, reduces hemorrhagic transformation and infarction volume, and ameliorates brain blood flow and neurological function in AIS mice. The mechanisms are associated with the activation of VIP/VIPR1 pathway to enhance BMECs viability and maintain tight junction phenotype.
Keywords: Acute ischemic stroke; Blood brain barrier; Shengui Sansheng San; Tissue plasminogen activator; VIP/VIPR1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors have declared no competing interests. Ethics approval and consent to participate: This experiment was approved by Ethics Committee of the University of Macau (APP-ARE-012). All procedures relating to the use of experimental animals are carried out strictly in accordance with the rules established by the University of Macau’s Animal Ethics Committee and the international NIH standards.
Figures



References
-
- Feigin VL, et al. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int J Stroke. 2022;17(1):18–29. - PubMed
-
- Pu L, et al. Projected global trends in ischemic stroke incidence, deaths and disability-adjusted life years from 2020 to 2030. Stroke. 2023;54(5):1330–9. - PubMed
-
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–7. - PubMed
-
- Katzan IL, et al. Utilization of intravenous tissue plasminogen activator for acute ischemic stroke. Arch Neurol. 2004;61(3):346–50. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

