The immunogenic potential of an optimized mRNA lipid nanoparticle formulation carrying sequences from virus and protozoan antigens
- PMID: 40102899
- PMCID: PMC11921523
- DOI: 10.1186/s12951-025-03201-8
The immunogenic potential of an optimized mRNA lipid nanoparticle formulation carrying sequences from virus and protozoan antigens
Abstract
Background: Lipid nanoparticles (LNP) are a safe and effective messenger RNA (mRNA) delivery system for vaccine applications, as shown by the COVID-19 mRNA vaccines. One of the main challenges faced during the development of these vaccines is the production of new and versatile LNP formulations capable of efficient encapsulation and delivery to cells in vivo. This study aimed to develop a new mRNA vaccine formulation that could potentially be used against existing diseases as well as those caused by pathogens that emerge every year.
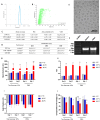
Results: Using firefly luciferase (Luc) as a reporter mRNA, we evaluated the physical-chemical properties, stability, and biodistribution of an LNP-mRNA formulation produced using a novel lipid composition and a microfluidic organic-aqueous precipitation method. Using mRNAs encoding a dengue virus or a Leishmania infantum antigen, we evaluated the immunogenicity of LNP-mRNA formulations and compared them with the immunization with the corresponding recombinant protein or plasmid-encoded antigens. For all tested LNP-mRNAs, mRNA encapsulation efficiency was higher than 85%, their diameter was around 100 nm, and their polydispersity index was less than 0.3. Following an intramuscular injection of 10 µg of the LNP-Luc formulation in mice, we detected luciferase activity in the injection site, as well as in the liver and spleen, as early as 6 h post-administration. LNPs containing mRNA encoding virus and parasite antigens were highly immunogenic, as shown by levels of antigen-specific IgG antibody as well as IFN-γ production by splenocytes of immunized animals that were similar to the levels that resulted from immunization with the corresponding recombinant protein or plasmid DNA.
Conclusions: Altogether, these results indicate that these novel LNP-mRNA formulations are highly immunogenic and may be used as novel vaccine candidates for different infectious diseases.
Keywords: Biodistribution; Dengue; IFNγ; IgG; Leishmaniasis; Lipid nanoparticles; mRNA vaccine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
-
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–11. - PubMed
-
- Hassett KJ, Benenato KE, Jacquinet E, Lee A, Woods A, Yuzhakov O, Himansu S, DeterLing J, Geilich BM, Ketova T, Mihai C, Lynn A, McFadyen I, Moore MJ, Senn JJ, Stanton MG, Almarsson O, Ciaramella G, Brito LA. Optimization of lipid nanoparticles for intramuscular administration of mrna vaccines. Mol Ther Nucleic Acids. 2019;15:1–11. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

