Wnt5a augments intracellular free cholesterol levels and promotes castration resistance in prostate cancer
- PMID: 40102900
- PMCID: PMC11921506
- DOI: 10.1186/s12967-025-06322-8
Wnt5a augments intracellular free cholesterol levels and promotes castration resistance in prostate cancer
Abstract
Background: Prostate cancer (PCa) is a leading cause of cancer-related mortality in men globally. While androgen deprivation therapy (ADT) can extend the asymptomatic phase and overall survival of patients with metastatic PCa, prolonged ADT often leads to the development of castration-resistant prostate cancer (CRPC) within 18-24 months. The mechanisms underlying CRPC remain incompletely understood, presenting a significant challenge in clinical prostate cancer treatment.
Methods: In this study, we investigated the role of Wnt5a, a member of the Wnt family, in CRPC. Tumor tissues from CRPC patients were analyzed to assess the expression levels of Wnt5a. Prostate cancer cells were used to examine the impact of Wnt5a on androgen-dependent and -independent growth, as well as sensitivity to bicalutamide. RNA-seq analysis, qRT-PCR, intracellular cholesterol content and the activation of the androgen receptor (AR) signaling pathway were evaluated to elucidate the mechanistic role of Wnt5a in CRPC progression. Drug target Mendelian randomization analysis was performed to investigate the effect of PCSK9 inhibitor on prostate cancer.
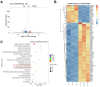
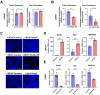
Results: Our study revealed a significant overexpression of Wnt5a in tissues from CRPC tumors. Wnt5a was found to enhance both androgen-dependent and -independent growth in prostate cancer cells while reducing their sensitivity to bicalutamide. Mechanistically, Wnt5a was shown to upregulate intracellular free cholesterol content and activate the AR signaling pathway, contributing to hormone therapy resistance in CRPC. PCSK9 inhibitor significantly reduced the risk of PCa.
Conclusions: The findings of this study highlight a novel molecular mechanism underlying endocrine therapy resistance in CRPC mediated by Wnt5a. Targeting Wnt5a or reducing cholesterol level would be a promising therapeutic strategy for the treatment of CRPC, providing new insights into potential avenues for combating this challenging form of prostate cancer.
Keywords: Androgen receptor signaling; Castration resistance; Cholesterol; Prostate cancer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: The study was reviewed and approved by the Ethics Committee of Ningxia Medical University. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures





References
-
- Onozawa M, Akaza H, Hinotsu S, Oya M, Ogawa O, Kitamura T, et al. Combined androgen Blockade achieved better oncological outcome in androgen deprivation therapy for prostate cancer: analysis of community-based multi-institutional database across Japan using propensity score matching.[J]. Cancer Med. 2018;7(10):4893–902. 10.1002/cam4.1735. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

