Development of the natural history component of an early economic model for primary sclerosing cholangitis
- PMID: 40102907
- PMCID: PMC11921552
- DOI: 10.1186/s13023-025-03658-8
Development of the natural history component of an early economic model for primary sclerosing cholangitis
Abstract
Background: Primary sclerosing cholangitis (PSC) is a rare, chronic cholestatic disease that can progress to cirrhosis and liver failure. The natural history of PSC is variable as liver enzymes and liver symptoms fluctuate over time. Several drugs for PSC are under investigation, but there are currently no economic models to evaluate the cost-effectiveness and value of new treatments. The objective of this study was to develop an early economic model for PSC and validate the natural history component.
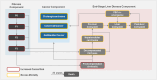
Methods: A lifetime horizon Markov cohort model was developed to track the progression of adults with PSC with or without inflammatory bowel disease. Based on relevant literature and clinical expert advice, fibrosis staging was used to model disease progression. Evidence on disease progression, mortality, PSC-related complications, and secondary cancers was identified by literature searches and validated by interviews with clinical and cost-effectiveness modelling experts. Model outcomes were overall survival and transplant-free survival years, and the proportions of patients receiving liver transplants, 2nd liver transplants after recurrent PSC (rPSC), and developing rPSC after liver transplantation during their lifetime. Cumulative incidence of secondary cancers and quality-adjusted life-years (QALYs) were also tracked.
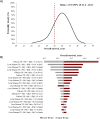
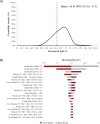
Results: Model outcomes are in line with estimates reported in literature recommended by clinical experts. Overall survival (95% uncertainty interval [UI]) was estimated to be 25.0 (23.2-26.3) years and transplant-free survival was estimated to be 22.0 (20.2-23.6) years. The estimated proportion (95% UI) of patients receiving first liver transplants was 14.5% (11.6-17.1%), while the proportion of patients developing rPSC and receiving 2nd liver transplants after rPSC was 24.2% (20.4-28.0%) and 21.6% (12.9-29.7%), respectively. The cumulative incidence (95% UI) of cholangiocarcinoma, colorectal cancer, and gallbladder cancer were estimated at 5.2% (2.1-10.0%), 3.6% (1.4-5.4%), and 3.3% (1.2-7.6%), respectively. Discounted lifetime QALYs per patient (95% UI) were estimated at 16.4 (15.6-17.1).
Conclusions: We have developed a model framework to simulate the progression of PSC with estimates of overall and transplant-free survival. This model, which calibrates well with existing estimates of disease progression, may be useful to evaluate the clinical and economic benefits of future treatments.
Keywords: Economic; Model; Natural history; Primary sclerosing cholangitis; Progression; Survival.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: CB has received grant funding and advisor fees from CymaBay Therapeutics, GSK, and Ipsen Bioscience; and has received grant funding from Boston Scientific, Calliditas Therapeutics, Cara Therapeutics, Chemomab, COUR Pharmaceuticals, Gilead Sciences, Hanmi Pharmaceuticals, Intercept Pharmaceuticals, Mirum Pharmaceuticals, Novo Nordisk, Pliant Therapeutics, and Viking Therapeutics; and has received advisor fees from Invea Therapeutics. CL has received grant funding and consultant fees from Calliditas Therapeutics, CymaBay Therapeutics, Gilead Sciences, GSK, Intercept Pharmaceuticals, Ipsen Bioscience, and Pliant Therapeutics; and has received grant funding from Cara Therapeutics, Escient Pharmaceuticals, GENFIT, HighTide Therapeutics, Mirum Pharmaceuticals, Novartis, and Zydus Lifesciences. KVK has received grant funding and consultant fees from CymaBay Therapeutics, GENFIT, Gilead Sciences, Intercept Pharmaceuticals, Madrigal Pharmaceuticals, Mirum Pharmaceuticals, NGM Biopharmaceuticals, Pfizer, and 89Bio; has received consultant fees from Enanta Pharmaceuticals, HighTide Therapeutics, and Inipharm; has received grant funding from Corcept Therapeutics, GSK, Hanmi Pharmaceutical, Novo Nordisk, Pliant Therapeutics, Terns Pharmaceuticals, and Viking Therapeutics; has received speaker bureau honoraria from AbbVie, Gilead Sciences, and Intercept Pharmaceuticals; and has stock options with Inipharm. NK is an employee of, and has stock options with, Gilead Sciences. SJ, YR, and NS are employees of Maple Health Group who conducted/executed the study on behalf of Gilead Sciences and were paid by Gilead Sciences for their services. AB and MS have received consultant fees from Gilead Sciences. DO has received consultant fees from Gilead Sciences and is employed at a research center that accepts sponsorship funding for its databases from life sciences companies, government agencies, and academic institutions.
Figures
Similar articles
-
Inflammatory conditions play a role in recurrence of PSC after liver transplantation: An international multicentre study.JHEP Rep. 2022 Oct 1;4(12):100599. doi: 10.1016/j.jhepr.2022.100599. eCollection 2022 Dec. JHEP Rep. 2022. PMID: 36426376 Free PMC article.
-
Recurrence of Primary Sclerosing Cholangitis and De Novo Cholangiocarcinoma After Liver Transplantation: Results From the Brazilian Cholestasis Consortium.Clin Transplant. 2024 Oct;38(10):e70002. doi: 10.1111/ctr.70002. Clin Transplant. 2024. PMID: 39436152
-
Risk factors for recurrent primary sclerosing cholangitis after liver transplantation.J Hepatol. 2015 Nov;63(5):1139-46. doi: 10.1016/j.jhep.2015.07.005. Epub 2015 Jul 14. J Hepatol. 2015. PMID: 26186988
-
Timing, Management, and Outcomes of Liver Transplantation in Primary Sclerosing Cholangitis.Semin Liver Dis. 2017 Nov;37(4):305-313. doi: 10.1055/s-0037-1608655. Epub 2017 Dec 22. Semin Liver Dis. 2017. PMID: 29272893 Review.
-
Role of colectomy in preventing recurrent primary sclerosing cholangitis in liver transplant recipients.World J Gastroenterol. 2018 Jul 28;24(28):3171-3180. doi: 10.3748/wjg.v24.i28.3171. World J Gastroenterol. 2018. PMID: 30065563 Free PMC article. Review.
References
-
- Barner-Rasmussen N, Pukkala E, Jussila A, Färkkilä M. Epidemiology, risk of malignancy and patient survival in primary sclerosing cholangitis: a population-based study in Finland. Scand J Gastroenterol. 2020;55:74–81. - PubMed
-
- Trivedi PJ, Bowlus CL, Yimam KK, Razavi H, Estes C, Epidemiology. Natural history, and outcomes of primary sclerosing cholangitis: A systematic review of population-based studies. Clin Gastroenterol Hepatol. 2022;20:1687– 700.e4. - PubMed

