The establishment and regulation of human germ cell lineage
- PMID: 40102947
- PMCID: PMC11921702
- DOI: 10.1186/s13287-025-04171-2
The establishment and regulation of human germ cell lineage
Abstract
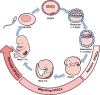
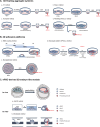
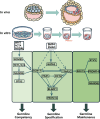
The specification of primordial germ cells (PGCs) during early embryogenesis initiates the development of the germ cell lineage that ensures the perpetuation of genetic and epigenetic information from parents to offspring. Defects in germ cell development may lead to infertility or birth defects. Historically, our understanding of human PGCs (hPGCs) regulation has primarily been derived from studies in mice, given the ethical restrictions and practical limitations of human embryos at the stage of PGC specification. However, recent studies have increasingly highlighted significant mechanistic differences for PGC development in humans and mice. The past decade has witnessed the establishment of human pluripotent stem cell (hPSC)-derived hPGC-like cells (hPGCLCs) as new models for studying hPGC fate specification and differentiation. In this review, we systematically summarize the current hPSC-derived models for hPGCLC induction, and how these studies uncover the regulatory machinery for human germ cell fate specification and differentiation, forming the basis for reconstituting gametogenesis in vitro from hPSCs for clinical applications and disease modeling.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors have declared that no competing interest exists. Statement on the use of AI: The authors declare that they have not use AI-generated work in this manuscript.
Figures
References
-
- Lesch BJ, Page DC. Genetics of germ cell development. Nat Rev Genet. 2012;13(11):781–94. - PubMed
-
- Extavour CG, Akam M. Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development. 2003;130(24):5869–84. - PubMed
-
- Santos AC, Lehmann R. Germ cell specification and Migration in Drosophila and beyond. Curr Biol. 2004;14(14):R578–89. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

