Efficacy and Hypoglycemia Profile of Once-weekly Insulin Icodec vs Once-daily Comparators Across Demographic Subgroups
- PMID: 40102962
- PMCID: PMC12527427
- DOI: 10.1210/clinem/dgaf168
Efficacy and Hypoglycemia Profile of Once-weekly Insulin Icodec vs Once-daily Comparators Across Demographic Subgroups
Abstract
Objective: This post hoc analysis evaluated the impact of age, ethnicity, and race on efficacy and hypoglycemia outcomes with once-weekly insulin icodec (icodec) vs once-daily (OD) basal insulin comparators, leveraging data from the ONWARDS 1 to 5 phase 3a clinical trials.
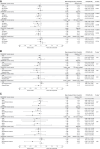
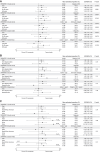
Methods: Efficacy and hypoglycemia outcomes were assessed within each trial in insulin-naive (ONWARDS 1, 3, and 5) and insulin-experienced (ONWARDS 2 and 4) adults (≥18 years) with type 2 diabetes across subgroups of age (<55, 55-64, and ≥65 years), ethnicity (Hispanic/Latino and non-Hispanic/Latino), and race (Asian, Black/African American, White, Other). The primary outcome was the change in glycated hemoglobin (HbA1c) from baseline to planned end of treatment. Other outcomes assessed included the achievement of HbA1c <7% (<53 mmol/mol) without clinically significant or severe hypoglycemia and the number of clinically significant or severe hypoglycemic episodes.
Results: Across all trials, the estimated treatment differences for change in HbA1c and the odds ratios for achieving HbA1c <7% (<53 mmol/mol) without clinically significant or severe hypoglycemia were similar across age, ethnicity, and race subgroups with icodec vs OD insulin (no statistically significant treatment by subgroup interactions were observed; P > .05 in all instances). Hypoglycemia rates were numerically low for both treatment groups and consistent across age, ethnicity, and race subgroups.
Conclusion: The efficacy and hypoglycemia profile of icodec vs OD comparators was consistent across trial populations irrespective of age, ethnicity, or race.
Keywords: demographic analyses; ethnicity; long-acting basal insulin; race; type 2 diabetes.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Endocrine Society.
Figures
References
-
- Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine (Baltimore). 2022;50(10):638‐643.
-
- Bellary S, Kyrou I, Brown JE, Bailey CJ. Type 2 diabetes mellitus in older adults: clinical considerations and management. Nat Rev Endocrinol. 2021;17(9):534‐548. - PubMed
-
- do Vale Moreira NC, Ceriello A, Basit A, et al. Race/ethnicity and challenges for optimal insulin therapy. Diabetes Res Clin Pract. 2021;175:108823. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

