Spatiotemporal perturbations of the plasminogen activation system in a rat model of acute organophosphate intoxication
- PMID: 40102979
- PMCID: PMC11917081
- DOI: 10.1186/s40478-025-01979-0
Spatiotemporal perturbations of the plasminogen activation system in a rat model of acute organophosphate intoxication
Abstract
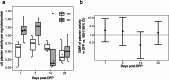
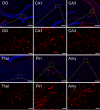
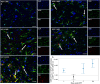
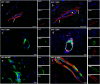
Neuroinflammation is widely posited to be a key pathogenic mechanism linking acute organophosphate (OP)-induced status epilepticus (SE) to persistent brain injury and abnormal electrical activity that contribute to epilepsy and cognitive impairment. The plasminogen activation system (PAS) promotes neuroinflammation in diverse neurological diseases but whether it is activated following acute OP intoxication has yet to be evaluated. To address this data gap, we characterized the spatiotemporal expression patterns of multiple components of the PAS in a rat model of acute intoxication with the OP, diisopropylfluorophosphate (DFP). Adult male Sprague Dawley rats administered DFP (4 mg/kg, sc), atropine sulfate (2 mg/kg, im) and 2-pralidoxime (25 mg/kg, im) went into SE that persisted for hours. One day after acute DFP-induced SE, plasmin activity and protein concentrations of plasminogen activator inhibitor-1 (PAI-1) in the plasma were increased, though not significantly. In contrast, acute DFP intoxication significantly increased brain levels of PAI-1, tissue-type plasminogen activator (tPA), urokinase plasminogen activator (uPA), and transcripts of TGF-β in a time- and region-dependent manner. In the cortex and hippocampus, quantification of PAI-1, tPA, and uPA by ELISA indicated significantly increased levels at 1 day post-exposure (DPE). PAI-1 and uPA returned to control values by 7 DPE while tPA protein remained elevated at 28 DPE. Immunohistochemistry detected elevated PAI-1 expression in the DFP brain up to 28 DPE. Co-localization of PAI-1 with biomarkers of neurons, microglia, and astrocytes demonstrated that PAI-1 localized predominantly to a subpopulation of astrocytes. Cytologically, PAI-1 localized to astrocytic end feet, but not adjacent neurovascular endothelium. Electron microscopy revealed neuronal metabolic stress and neurodegeneration with disruption of adjacent neurovascular units in the hippocampus post-DFP exposure. These data indicate that acute DFP intoxication altered PAS expression in the brain, with aberrant PAI-1 expression in a subset of reactive astrocyte populations.
Keywords: Blood-brain barrier; Diisopropylfluorophosphate; Epilepsy; Neuroinflammation; Plasminogen activator inhibitor-1 (PAI-1).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: Animals were maintained in facilities fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), and all studies were performed with regard to the alleviation of pain and suffering under protocols approved by the UC Davis Institutional Animal Care and Use Committee (IACUC protocol numbers 201865, 201954). Animal experiments were conducted in accordance with ARRIVE guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Consent to Publish: Not applicable; no human subjects involved. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Persistent neuroinflammation and cognitive impairment in a rat model of acute diisopropylfluorophosphate intoxication.J Neuroinflammation. 2016 Oct 12;13(1):267. doi: 10.1186/s12974-016-0744-y. J Neuroinflammation. 2016. PMID: 27733171 Free PMC article.
-
Persistent behavior deficits, neuroinflammation, and oxidative stress in a rat model of acute organophosphate intoxication.Neurobiol Dis. 2020 Jan;133:104431. doi: 10.1016/j.nbd.2019.03.019. Epub 2019 Mar 21. Neurobiol Dis. 2020. PMID: 30905768 Free PMC article. Review.
-
Diet-incorporated saracatinib, a Src tyrosine kinase inhibitor, counteracts diisopropylfluorophosphate (DFP)-induced chronic neurotoxicity in the rat model.Biomed Pharmacother. 2025 Aug;189:118234. doi: 10.1016/j.biopha.2025.118234. Epub 2025 Jun 12. Biomed Pharmacother. 2025. PMID: 40513390 Free PMC article.
-
Invasion and metastasis of hepatocellular carcinoma in relation to urokinase-type plasminogen activator, its receptor and inhibitor.J Cancer Res Clin Oncol. 2000 Nov;126(11):641-6. doi: 10.1007/s004320000146. J Cancer Res Clin Oncol. 2000. PMID: 11079728 Free PMC article.
-
NTP Developmental and Reproductive Toxicity Technical Report on the Prenatal Development Studies of 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine (CASRN 95737-68-1) in Sprague Dawley (Hsd:Sprague Dawley® SD®) Rats and New Zealand White (Hra:NZW SPF) Rabbits: DART Report 07 [Internet].Research Triangle Park (NC): National Toxicology Program; 2022 Jan. Research Triangle Park (NC): National Toxicology Program; 2022 Jan. PMID: 35593777 Free Books & Documents. Review.
References
-
- Chen Y (2012) Organophosphate-induced brain damage: mechanisms, neuropsychiatric and neurological consequences, and potential therapeutic strategies. Neurotoxicology 33:391–400. 10.1016/j.neuro.2012.03.011 - PubMed
-
- Loh Y, Swanberg MM, Ingram MV, Newmark J (2010) Case report: Long-term cognitive sequelae of sarin exposure. Neurotoxicology 31:244–246. 10.1016/j.neuro.2009.12.004 - PubMed
-
- Ohbu S, Yamashina A, Takasu N, Yamaguchi T, Murai T et al (1997) Sarin poisoning on Tokyo subway. South Med J 90:587–593. 10.1097/00007611-199706000-00002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

