Improving effects of melatonin on memory and synaptic potentiation in a mouse model of Alzheimer's-like disease: the involvement of glutamate homeostasis and mGluRs receptors
- PMID: 40102986
- PMCID: PMC11916854
- DOI: 10.1186/s12993-025-00271-4
Improving effects of melatonin on memory and synaptic potentiation in a mouse model of Alzheimer's-like disease: the involvement of glutamate homeostasis and mGluRs receptors
Abstract
Background: Alzheimer's disease (AD) is characterized by progressive cognitive decline and synaptic dysfunction, largely driven by amyloid plaques and neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau. These pathological hallmarks disrupt glutamate signaling, which is essential for synaptic plasticity and memory consolidation. This study investigates the therapeutic potential of melatonin on memory and synaptic plasticity in an AD-like mouse model, with a focus on its regulatory effects on glutamate homeostasis and metabotropic glutamate receptors (mGluRs).
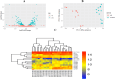
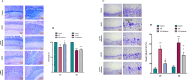
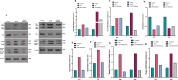
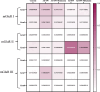
Methods: The study began with an in-silico bioinformatics analysis of RNA-seq datasets from hippocampal tissues of AD patients to identify differentially expressed genes (DEGs) related to glutamate signaling and tau pathology. An AD-like model was induced via intra-hippocampal injection of cis-phospho tau in C57BL/6 mice. Memory function was assessed using behavioral tests. Synaptic plasticity was evaluated using in vitro field potential recording of hippocampal slices. Histological analyses included Nissl staining for neuronal density, Luxol Fast Blue for myelin integrity, and immunofluorescence for tau hyperphosphorylation. Molecular studies employed qPCR and Western blot to assess glutamate-related markers and tau phosphorylation. Melatonin (10 mg/kg) was administered intraperitoneally, starting either two weeks (early intervention) or four weeks (late intervention) post-induction.
Results: Key molecular targets in glutamate signaling pathways were identified using bioinformatics. AD-like mice displayed memory deficits and synaptic dysfunction. Melatonin improved cognitive function, especially with early intervention, as confirmed by behavioral tests. Histological studies revealed reduced neuronal loss, improved myelin integrity, and decreased tau hyperphosphorylation. Molecular findings showed restored mGluR expression and reduced GSK3 activity. Early intervention yielded superior outcomes, with partial restoration of synaptic plasticity observed in LTP recordings.
Conclusions: These findings underscore the neuroprotective properties of melatonin, mediated by its ability to modulate glutamate signaling and mGluR activity, offering new insights into its potential as a therapeutic agent for AD. Additionally, the results suggest that earlier administration of melatonin may significantly enhance its efficacy, highlighting the importance of timely intervention in neurodegenerative diseases.
Keywords: Alzheimer's disease; Cis-phospho tau; Glutamate signaling; Melatonin; Memory; Metabotropic glutamate receptors; Synaptic plasticity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All experiments were conducted following the Guidelines for the care and use of laboratory animals and approved by the “Ethics Committee of Tarbiat Modares University” (IR.MODARES.REC.1400.217). Consent for publication: Not applicable Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
Troriluzole rescues glutamatergic deficits, amyloid and tau pathology, and synaptic and memory impairments in 3xTg-AD mice.J Neurochem. 2025 Jan;169(1):e16215. doi: 10.1111/jnc.16215. Epub 2024 Aug 30. J Neurochem. 2025. PMID: 39214859
-
Exploring Dimethylsulfoniopropionate as a potential treatment for Alzheimer's disease: A study using the 3 × Tg-AD mouse model.Phytomedicine. 2024 Jul 25;130:155788. doi: 10.1016/j.phymed.2024.155788. Epub 2024 May 28. Phytomedicine. 2024. PMID: 38838634
-
Melatonin ameliorates amyloid beta-induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI3/Akt/GSk3β pathway in the mouse hippocampus.J Pineal Res. 2015 Aug;59(1):47-59. doi: 10.1111/jpi.12238. Epub 2015 May 12. J Pineal Res. 2015. PMID: 25858697
-
Metabotropic Glutamate Receptors in Alzheimer's Disease Synaptic Dysfunction: Therapeutic Opportunities and Hope for the Future.J Alzheimers Dis. 2020;78(4):1345-1361. doi: 10.3233/JAD-201146. J Alzheimers Dis. 2020. PMID: 33325389 Free PMC article. Review.
-
Melatonin in Alzheimer's Disease: A Latent Endogenous Regulator of Neurogenesis to Mitigate Alzheimer's Neuropathology.Mol Neurobiol. 2019 Dec;56(12):8255-8276. doi: 10.1007/s12035-019-01660-3. Epub 2019 Jun 17. Mol Neurobiol. 2019. PMID: 31209782 Review.
Cited by
-
Psychoactive substances: novel molecular insights and therapeutic potential for Alzheimer's disease.Transl Neurodegener. 2025 Jul 25;14(1):38. doi: 10.1186/s40035-025-00498-1. Transl Neurodegener. 2025. PMID: 40713680 Free PMC article. Review.
-
The Novel Melatonin Analog Containing Donepezil Fragment Prevents Cognitive Impairments and Associated Oxidative Stress in a Hybrid Rat Model of Melatonin Deficiency and icvAβ1-42.Int J Mol Sci. 2025 Jul 8;26(14):6553. doi: 10.3390/ijms26146553. Int J Mol Sci. 2025. PMID: 40724803 Free PMC article.
References
-
- Colom-Cadena M, Davies C, Sirisi S, Lee J-E, Simzer EM, Tzioras M, et al. Synaptic oligomeric tau in Alzheimer’s disease — A potential culprit in the spread of tau pathology through the brain. Neuron. 2023; https://linkinghub.elsevier.com/retrieve/pii/S0896627323003057 - PubMed
-
- Simons M, Levin J, Dichgans M. Tipping points in neurodegeneration. Neuron. 2023. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

