Hypoxemia in trauma patients receiving two different oxygen strategies: a TRAUMOX2 substudy
- PMID: 40102987
- PMCID: PMC11921562
- DOI: 10.1186/s13049-025-01360-z
Hypoxemia in trauma patients receiving two different oxygen strategies: a TRAUMOX2 substudy
Abstract
Background: The randomized controlled trial, TRAUMOX2, compared early restrictive vs. liberal oxygen strategies for trauma patients. The objective of this substudy was to quantify the occurrence and duration of hypoxemic episodes during the trial's eight-hour intervention.
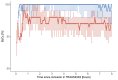
Methods: This observational substudy analyzed a subset of patients at two trial sites in Denmark. Continuous pulse oximetry recorded arterial oxygen saturation (SpO2) during the intervention. The primary outcome was the proportion of patients who had episodes of hypoxemia with SpO2 < 90% for at least five minutes. Additionally, the study assessed differences in the occurrence and duration of hypoxemia between the restrictive and liberal oxygen groups.
Results: This substudy included 82 patients. After secondary exclusion, 60 patients (median age, 49 years [interquartile range 33-61] and 75% male) were analyzed. Three out of 60 patients (5%) had at least one episode of SpO2 < 90% for at least five minutes (95% confidence interval 1-14%); Two patients in the restrictive oxygen group and one in the liberal oxygen group. Two episodes occurred during initial resuscitation, and one episode occurred in the intensive care unit following a procedure related to thoracic injuries.
Conclusions: In this substudy of 60 patients from the TRAUMOX2 trial, hypoxemia (SpO2 < 90% for at least five minutes) was observed in 5% of patients, with no difference between the restrictive and liberal oxygen groups. These findings suggest that, among trauma patients not already requiring continuous monitoring, such episodes of hypoxemia are relatively rare early post-trauma.
Keywords: Denmark; Hypoxemia; Oxygen therapy; Pulse oximetry; TRAUMOX2; Trauma.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The Danish Research Ethics Committee (H-21018062) and the Danish Medicines Agency (EudraCT 2021-000556-19) approved the TRAUMOX2 trial, which was also registered in the Capital Region of Denmark data controller’s register (P-2021-476), and at ClinicalTrials.gov (NCT05146700). Additional approvals, not relevant to this substudy, were also obtained. Consent for publication: Not applicable. Competing interests: Tobias Arleth: Received funding for a biomarker Copenhagen substudy of TRAUMOX2 from the Holger and Ruth Hesses Memorial Foundation and Danish Air Ambulance, and received funding for three months of research PhD programme exchange from Knud Højgaard’s Foundation, the William Demant Foundation, and Ottilia Brorson’s Travel Scholarships, the Medical Science Faculty Foundation of Copenhagen University, and the Consultant Dr. Med. Edgar Schnohr and wife Gilberte Schnohrs Foundation. All grants were received outside the submitted work. Josefine Baekgaard: Received funding for the TRAUMOX2 trial from the Novo Nordisk Foundation. She was also awarded a prize for “Talented Young Researcher” by the Lundbeck Foundation from which €47,000 was allocated to research, with some of the money spent on this trial. Stine Zwisler: Attended a Secma ultrasound course in Italy (March 2024), where Secma paid for the trip, and no payment was made to her or her organization. Markus Klimek: Received grants to organization for research laboratory Music as Medicine; honoraria from Paion as a member of the data safety board and Tijdstroom Uitgeverij for serving as an editor of a Dutch textbook; and serving as a course director for Advanced Trauma Life Support outside the submitted work. Jacob Steinmetz: Received funding of his professorship to his institution from the Norwegian Air Ambulance foundation. The remaining authors (OR, AC, LBP, SM, LSR) declare to have potential conflicts of interest.
Figures

References
-
- American College of Surgeons. The committee on trauma. ATLS: advanced trauma.Life support (Student course Manual). 10th ed. Chicago: Am Coll Surg; 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

