Allogeneic hematopoietic stem cell transplantation for mucopolysaccharidosis patients: a single-center experience and assessment of quality of life
- PMID: 40102994
- PMCID: PMC11921649
- DOI: 10.1186/s13052-025-01919-7
Allogeneic hematopoietic stem cell transplantation for mucopolysaccharidosis patients: a single-center experience and assessment of quality of life
Abstract
Background: Allogeneic hematopoietic stem cell transplantation (HSCT) has proven to be a viable treatment option for patients with mucopolysaccharidoses (MPS). We investigate the efficacy and improvements in the quality of life of HSCT in pediatric patients with MPS.
Methods: A retrospective analysis of transplantation data from 46 cases of MPS from a single institution in China was conducted.
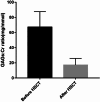
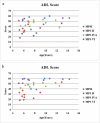
Results: The cohort of 46 patients included 9 cases of MPS I, 16 cases of MPS II, 15 cases of MPS IVA and 6 cases of MPS VI. The median age at diagnosis was 2.59 years. The median age at transplantation was 3.80 years. The median follow-up time was 3.1 years (range, 0.8-8.1 years) and 43 patients were alive. The incidence of grades II to IV aGVHD was 17.4%, wherein the incidence of grades III and IV aGVHD was 4.3%. The incidence of moderate-to-severe cGVHD was 6.5%. GAGs urinary excretion decreased and enzyme activity levels reached normal. After HSCT, multiple bone dysplasia, upper-airway obstruction and recurrent otitis media were significantly improved; vision, corneal clouding, cardiovascular disease, hepatosplenomegaly and hydrocephalus were improved or remained stable; neurological symptoms were improved or remained stable in most patients but progressed in others; the patients with MPS IH/S and MPS II reached nearly normal growth rate of height and weight. Meanwhile, the patients with MPS IH, MPS IVA and MPS VI remained poor growth after HSCT. The Activities of Daily Living (ADL) scores were improved in most patients with MPS. ADL scores in patients with severe phenotypes were lower than health control subjects and patients with attenuated phenotypes.
Conclusions: HSCT is a good therapeutic option for MPS and improves the quality of life of patients. MPS patients with attenuated phenotypes provide a better outcome in ADL after HSCT.
Keywords: Allogeneic hematopoietic stem cell transplantation; Mucopolysaccharidosis; Outcomes; Quality of life.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Institutional Review Board of Guangzhou Women and Children’s Medical Center and all methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from the legal guardians of all the participants in the study. Consent for publication: Institutional consent form has been used to obtain the consent to publication from children’s parents or legal guardian. Competing interests: The authors declare that they have no competing interests. The study was carried out according to the amended Declaration of Helsinki.
Figures

Similar articles
-
Allogeneic Hematopoietic Stem Cell Transplantation in Thirty-Four Pediatric Cases of Mucopolysaccharidosis-A Ten-Year Report from the China Children Transplant Group.Biol Blood Marrow Transplant. 2016 Nov;22(11):2104-2108. doi: 10.1016/j.bbmt.2016.08.015. Epub 2016 Aug 20. Biol Blood Marrow Transplant. 2016. PMID: 27555533
-
Hematopoietic stem cell transplantation in children with mucopolysaccharidosis IVA: single center experience.Bone Marrow Transplant. 2025 Jan;60(1):47-51. doi: 10.1038/s41409-024-02439-4. Epub 2024 Oct 14. Bone Marrow Transplant. 2025. PMID: 39402187
-
Long-Term Outcomes of Hematopoietic Stem Cell Transplantation in Mucopolysaccharidoses Patients Without Radiation.Clin Transplant. 2025 Jun;39(6):e70188. doi: 10.1111/ctr.70188. Clin Transplant. 2025. PMID: 40509920
-
Hematopoietic Stem Cell Transplantation for Mucopolysaccharidoses: Past, Present, and Future.Biol Blood Marrow Transplant. 2019 Jul;25(7):e226-e246. doi: 10.1016/j.bbmt.2019.02.012. Epub 2019 Feb 14. Biol Blood Marrow Transplant. 2019. PMID: 30772512 Free PMC article. Review.
-
Mucopolysaccharidosis Type I.2002 Oct 31 [updated 2024 Apr 11]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2002 Oct 31 [updated 2024 Apr 11]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301341 Free Books & Documents. Review.
Cited by
-
CRISPR/nCas9-Edited CD34+ Cells Rescue Mucopolysaccharidosis IVA Fibroblasts Phenotype.Int J Mol Sci. 2025 May 2;26(9):4334. doi: 10.3390/ijms26094334. Int J Mol Sci. 2025. PMID: 40362571 Free PMC article.
References
-
- Chen HH, Sawamoto K, Mason RW, Kobayashi H, Yamaguchi S, Suzuki Y, et al. Enzyme replacement therapy for mucopolysaccharidoses; past, present, and future. J Hum Genet. 2019;64(11):1153–71. - PubMed
-
- Sawamoto K, Stapleton M, Alméciga-Díaz CJ, Espejo-Mojica AJ, Losada JC, Suarez DA, et al. Therapeutic options for mucopolysaccharidoses: current and emerging treatments. Drugs. 2019;79(10):1103–34. - PubMed
MeSH terms
Grants and funding
- 202102080028/Guangzhou Municipal Science and Technology Project
- 2022A1515012524/Natural Science Foundation of Guangdong Province
- NKE-PRE-2019-003/Guangzhou Institute of Pediatrics, Guangzhou Women and Childrens Medical Center
- YIP-2016-010/Guangzhou Institute of Pediatrics, Guangzhou Women and Childrens Medical Center
- K-202108-5/Guangdong Medical Research Foundation
LinkOut - more resources
Full Text Sources

