A new level of RNA-based plant protection: dsRNAs designed from functionally characterized siRNAs highly effective against Cucumber mosaic virus
- PMID: 40103224
- PMCID: PMC11904787
- DOI: 10.1093/nar/gkaf136
A new level of RNA-based plant protection: dsRNAs designed from functionally characterized siRNAs highly effective against Cucumber mosaic virus
Abstract
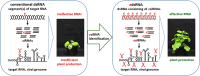
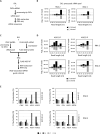
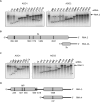
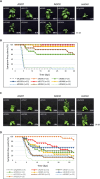
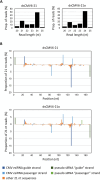
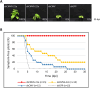
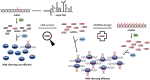
RNA-mediated crop protection increasingly becomes a viable alternative to agrochemicals that threaten biodiversity and human health. Pathogen-derived double-stranded RNAs (dsRNAs) are processed into small interfering RNAs (siRNAs), which can then induce silencing of target RNAs, e.g. viral genomes. However, with currently used dsRNAs, which largely consist of undefined regions of the target RNAs, silencing is often ineffective: processing in the plant generates siRNA pools that contain only a few functionally effective siRNAs (esiRNAs). Using an in vitro screen that reliably identifies esiRNAs from siRNA pools, we identified esiRNAs against Cucumber mosaic virus (CMV), a devastating plant pathogen. Topical application of esiRNAs to plants resulted in highly effective protection against massive CMV infection. However, optimal protection was achieved with newly designed multivalent 'effective dsRNAs' (edsRNAs), which contain the sequences of several esiRNAs and are preferentially processed into these esiRNAs. The esiRNA components can attack one or more target RNAs at different sites, be active in different silencing complexes, and provide cross-protection against different viral variants-important properties for combating rapidly mutating pathogens such as CMV. esiRNAs and edsRNAs have thus been established as a new class of 'RNA actives' that significantly increase the efficacy and specificity of RNA-mediated plant protection.
Plain language summary
RNA-mediated crop protection offers a promising alternative to harmful agrochemicals by using pathogen-derived double-stranded RNAs (dsRNAs) that are processed into small interfering RNAs (siRNAs) in plants. These siRNAs can silence viral genomes, but current dsRNAs are often ineffective. The authors have developed a method to identify effective siRNAs (esiRNAs) against the Cucumber mosaic virus, providing strong protection. Even better results were achieved with ‘effective dsRNAs’, which contain multiple esiRNAs and target various viral strains. This new class of ‘RNA actives’ significantly improves the effectiveness and precision of RNA-based plant protection.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Conflict of interest statement
None declared.
Figures

Similar articles
-
Highly efficacious antiviral protection of plants by small interfering RNAs identified in vitro.Nucleic Acids Res. 2019 Sep 26;47(17):9343-9357. doi: 10.1093/nar/gkz678. Nucleic Acids Res. 2019. PMID: 31433052 Free PMC article.
-
Topical Application of Double-Stranded RNA Targeting 2b and CP Genes of Cucumber mosaic virus Protects Plants against Local and Systemic Viral Infection.Plants (Basel). 2021 May 12;10(5):963. doi: 10.3390/plants10050963. Plants (Basel). 2021. PMID: 34066062 Free PMC article.
-
A viral satellite RNA induces yellow symptoms on tobacco by targeting a gene involved in chlorophyll biosynthesis using the RNA silencing machinery.PLoS Pathog. 2011 May;7(5):e1002021. doi: 10.1371/journal.ppat.1002021. Epub 2011 May 5. PLoS Pathog. 2011. PMID: 21573143 Free PMC article.
-
Exogenous RNAs for Gene Regulation and Plant Resistance.Int J Mol Sci. 2019 May 8;20(9):2282. doi: 10.3390/ijms20092282. Int J Mol Sci. 2019. PMID: 31072065 Free PMC article. Review.
-
Tuning Beforehand: A Foresight on RNA Interference (RNAi) and In Vitro-Derived dsRNAs to Enhance Crop Resilience to Biotic and Abiotic Stresses.Int J Mol Sci. 2021 Jul 19;22(14):7687. doi: 10.3390/ijms22147687. Int J Mol Sci. 2021. PMID: 34299307 Free PMC article. Review.
Cited by
-
Engineered Biomolecular Condensates Limit Tobacco Mosaic Virus Accumulation and Symptom Development.Mol Plant Pathol. 2025 Jun;26(6):e70113. doi: 10.1111/mpp.70113. Mol Plant Pathol. 2025. PMID: 40544335 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

