The phytochrome B signaling regulates salt-mediated seedling growth in the dark
- PMID: 40103321
- PMCID: PMC12125576
- DOI: 10.1093/pcp/pcaf029
The phytochrome B signaling regulates salt-mediated seedling growth in the dark
Abstract
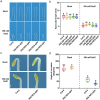
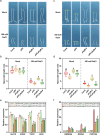
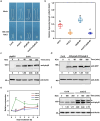
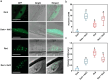
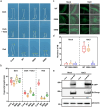
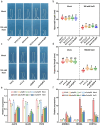
Light is an essential environmental factor that facilitates the robust upward growth of post-germinative seedlings emerging from buried seeds that is partly mediated by the photoreceptors. Salinity stress hampers plant growth and development and reduces yield. However, the involvement and regulatory role of photoreceptors and light signaling factors to salt stress are largely unknown. Here, we report that mutants of the phytochrome B (phyB) photoreceptor showed reduced sensitivity to salt-inhibited hypocotyl elongation in darkness, and that PHYTOCHROME-INTERACTING FACTOR 3 (PIF3) acts downstream of phyB in regulating this process in Arabidopsis thaliana. We also show that SALT OVERLY SENSITIVE 2 (SOS2) regulates phyB protein accumulation under salt stress in darkness. Surprisingly, salt treatment induces phyB nuclear body formation in darkness. Moreover, we found that the phosphorylation at residue Ser-86 of phyB is essential for its function, and the scaffold protein 14-3-3κ is involved in the regulation of phyB under salt stress in darkness. Taken together, our study reveals a regulatory role of the phyB-PIF3 module in mediating post-germination growth in darkness in response to salt stress.
Keywords: 14-3-3; SOS2; etiolated seedling; phyB; salt stress.
© The Author(s) 2025. Published by Oxford University Press on behalf of Japanese Society of Plant Physiologists.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Phytochrome-imposed oscillations in PIF3 protein abundance regulate hypocotyl growth under diurnal light/dark conditions in Arabidopsis.Plant J. 2012 Aug;71(3):390-401. doi: 10.1111/j.1365-313X.2012.04992.x. Epub 2012 Jun 11. Plant J. 2012. PMID: 22409654 Free PMC article.
-
The basic helix-loop-helix transcription factor PIF5 acts on ethylene biosynthesis and phytochrome signaling by distinct mechanisms.Plant Cell. 2007 Dec;19(12):3915-29. doi: 10.1105/tpc.107.051508. Epub 2007 Dec 7. Plant Cell. 2007. PMID: 18065691 Free PMC article.
-
A cytosol-tethered YHB variant of phytochrome B retains photomorphogenic signaling activity.Plant Mol Biol. 2024 Jun 14;114(4):72. doi: 10.1007/s11103-024-01469-2. Plant Mol Biol. 2024. PMID: 38874897 Free PMC article.
-
Modulation of warm temperature-sensitive growth using a phytochrome B dark reversion variant, phyB[G515E], in Arabidopsis and rice.J Adv Res. 2024 Sep;63:57-72. doi: 10.1016/j.jare.2023.11.001. Epub 2023 Nov 4. J Adv Res. 2024. PMID: 37926145 Free PMC article.
-
Phytochrome signaling in green Arabidopsis seedlings: impact assessment of a mutually negative phyB-PIF feedback loop.Mol Plant. 2012 May;5(3):734-49. doi: 10.1093/mp/sss031. Epub 2012 Apr 5. Mol Plant. 2012. PMID: 22492120 Free PMC article.
References
-
- Ali A, Petrov V, Yun D and Gechev T (2023) Revisiting plant salt tolerance: novel components of the SOS pathway. Trends Plant Sci. 28: 1060–1069. - PubMed
-
- Alonso J.M., Stepanova A.N., Leisse T.J., Kim C.J., Chen H., Shinn P., et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. - PubMed
-
- Al-Sady B., Ni W., Kircher S., Schafer E. and Quail P.H. (2006) Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol. Cell 23: 439–446. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

