Discrepancies in Dapagliflozin Response in Terms of Glycemic Control and Body Weight Reduction
- PMID: 40103330
- PMCID: PMC12061751
- DOI: 10.3803/EnM.2024.2142
Discrepancies in Dapagliflozin Response in Terms of Glycemic Control and Body Weight Reduction
Abstract
Backgruound: Dapagliflozin, a sodium-glucose cotransporter 2 inhibitor, reduces hyperglycemia and obesity by inhibiting renal glucose reabsorption. This post hoc study evaluated clinical factors influencing patient response to dapagliflozin.
Methods: The analysis focused on patients treated with dapagliflozin (10 mg/day for 52 weeks) within the randomized, double-blind, parallel-group BEYOND trial. Adequate glycemic control (GC) was defined as a reduction in glycated hemoglobin (HbA1c) of ≥ 1.0% or the achievement of an HbA1c level <7.0% at week 52. Significant weight loss (WL) referred to a reduction in body weight of ≥3.0% at week 52. Participants were classified into four groups based on their GC and WL responses: GC+/WL+, GC+/WL-, GC-/WL+, and GC-/WL-.
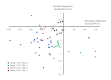
Results: Among dapagliflozin recipients (n=56), at 52 weeks, HbA1c had decreased by 1.0%±0.8% from baseline, while body weight had declined by 2.4±3.1 kg. Overall, 69.6% of participants achieved GC+, and 57.1% achieved WL+. Male sex and shorter diabetes duration were significantly associated with achieving GC+. Conversely, higher estimated glomerular filtration rate was significantly linked to WL+. The only factor significantly associated with both GC+ and WL+ was shorter diabetes duration (odds ratio, 0.81; 95% confidence interval, 0.68 to 0.97; P=0.023). The GC+ and WL+ groups exhibited favorable responses beginning soon after dapagliflozin therapy was initiated. Furthermore, HbA1c decline was more strongly associated with reduction in visceral fat than with WL.
Conclusion: A short duration of diabetes and early response to treatment appear to represent key factors in maximizing the benefits of dapagliflozin for blood glucose and weight management.
Keywords: Dapagliflozin; Diabetes mellitus, type 2; Effectiveness; Sodium-glucose transporter 2 inhibitors.
Conflict of interest statement
This work was financially supported by AstraZeneca Korea. The company funder had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the article for publication.
Figures
Similar articles
-
Efficacy of dapagliflozin versus sitagliptin on cardiometabolic risk factors in Japanese patients with type 2 diabetes: a prospective, randomized study (DIVERSITY-CVR).Cardiovasc Diabetol. 2020 Jan 7;19(1):1. doi: 10.1186/s12933-019-0977-z. Cardiovasc Diabetol. 2020. PMID: 31910850 Free PMC article. Clinical Trial.
-
Dapagliflozin as monotherapy in drug-naive Asian patients with type 2 diabetes mellitus: a randomized, blinded, prospective phase III study.Clin Ther. 2014 Jan 1;36(1):84-100.e9. doi: 10.1016/j.clinthera.2013.11.002. Epub 2013 Dec 28. Clin Ther. 2014. PMID: 24378206 Clinical Trial.
-
[Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin].Dtsch Med Wochenschr. 2013 Apr;138 Suppl 1:S6-15. doi: 10.1055/s-0032-1305283. Epub 2013 Mar 25. Dtsch Med Wochenschr. 2013. PMID: 23529570 Clinical Trial. German.
-
[Dapagliflozin, the first SGLT-2 inhibitor in the treatment of type 2 diabetes].Med Clin (Barc). 2013 Sep;141 Suppl 2:36-43. doi: 10.1016/S0025-7753(13)70062-9. Med Clin (Barc). 2013. PMID: 24444523 Review. Spanish.
-
Efficacy and safety of dapagliflozin as monotherapy in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials.Medicine (Baltimore). 2019 Jul;98(30):e16575. doi: 10.1097/MD.0000000000016575. Medicine (Baltimore). 2019. PMID: 31348290 Free PMC article.
References
-
- Horie I, Abiru N, Hongo R, Nakamura T, Ito A, Haraguchi A, et al. Increased sugar intake as a form of compensatory hyperphagia in patients with type 2 diabetes under dapagliflozin treatment. Diabetes Res Clin Pract. 2018;135:178–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

