Contrasting effects of mismatch locations on Z-DNA formation under bending force
- PMID: 40103725
- PMCID: PMC11912502
- DOI: 10.1039/d5sc00749f
Contrasting effects of mismatch locations on Z-DNA formation under bending force
Abstract
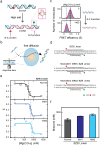
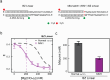
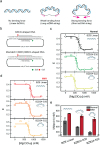
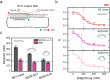
Z-DNA is a non-canonical, left-handed helical structure that plays crucial roles in various cellular processes. DNA mismatches, which involve the incorporation of incorrect Watson-Crick base pairs, are present in all living organisms and contribute to the mechanism of Z-DNA formation. However, the impact of mismatches on Z-DNA formation remains poorly understood. Moreover, the combined effect of DNA mismatches and bending, a common biological phenomenon observed in vivo, has not yet been explored due to technological limitations. Here, using single-molecule FRET, we show that a mismatch inside the Z-DNA region, i.e., the CG repeat region, hinders Z-DNA formation. In stark contrast, however, a mismatch in the B-Z junction facilitates Z-DNA formation. When the bending force is applied on double stranded DNA, a mismatch in the B-Z junction releases the bending stress more effectively than one in the CG repeat region. These findings provide mechanical insights into the role of DNA mismatches and bending forces in regulating Z-DNA formation, whether promoting or inhibiting it in biological environments.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
DNA Bending Force Facilitates Z-DNA Formation under Physiological Salt Conditions.J Am Chem Soc. 2022 Jul 27;144(29):13137-13145. doi: 10.1021/jacs.2c02466. Epub 2022 Jul 15. J Am Chem Soc. 2022. PMID: 35839423 Free PMC article.
-
Sequence dependent influence of an A…A mismatch in a DNA duplex: An insight into the recognition by hZαADAR1 protein.J Struct Biol. 2021 Mar;213(1):107678. doi: 10.1016/j.jsb.2020.107678. Epub 2020 Dec 8. J Struct Biol. 2021. PMID: 33307177
-
Twisting right to left: A…A mismatch in a CAG trinucleotide repeat overexpansion provokes left-handed Z-DNA conformation.PLoS Comput Biol. 2015 Apr 13;11(4):e1004162. doi: 10.1371/journal.pcbi.1004162. eCollection 2015 Apr. PLoS Comput Biol. 2015. PMID: 25876062 Free PMC article.
-
Toward an Expanded Genome: Structural and Computational Characterization of an Artificially Expanded Genetic Information System.Acc Chem Res. 2017 Jun 20;50(6):1375-1382. doi: 10.1021/acs.accounts.6b00655. Epub 2017 Jun 8. Acc Chem Res. 2017. PMID: 28594167 Free PMC article. Review.
-
Watson-Crick versus Hoogsteen Base Pairs: Chemical Strategy to Encode and Express Genetic Information in Life.Acc Chem Res. 2021 May 4;54(9):2110-2120. doi: 10.1021/acs.accounts.0c00734. Epub 2021 Feb 16. Acc Chem Res. 2021. PMID: 33591181 Review.
References
LinkOut - more resources
Full Text Sources

