Nitrene-mediated aminative N-N-N coupling: facile access to triazene 1-oxides
- PMID: 40103730
- PMCID: PMC11912504
- DOI: 10.1039/d5sc00064e
Nitrene-mediated aminative N-N-N coupling: facile access to triazene 1-oxides
Abstract
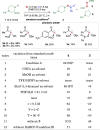
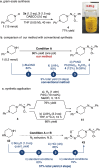
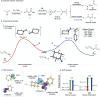
Significant progress has been made in metal-catalyzed cross-coupling reactions over the past few decades. However, the development of innovative aminative coupling strategies remains highly desirable. Herein, we report a nitrene-mediated aminative N-N-N coupling reaction that leverages an anomeric amide as a key reagent to bridge amines with nitrosoarenes. This strategy enables the in situ generation of an aminonitrene intermediate, which is efficiently intercepted by nitrosoarenes, providing a direct, mild, and highly efficient route to triazene 1-oxides. Mechanistic investigations reveal that the N-substituents of the amine play a crucial role in modulating the reactivity of the aminonitrene intermediate. Complementary computational studies further indicate that aminonitrene acts as a nucleophile, while nitrosobenzene serves as an electrophile. Notably, aminonitrene-nitrosoarene coupling is significantly favored due to a substantial reduction in distortion energy, effectively outcompeting the nitrene dimerization pathway.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Anomeric Amide-Enabled Alkene-Arene and Alkene-Alkene Aminative Coupling.Angew Chem Int Ed Engl. 2025 Jan 27;64(5):e202418141. doi: 10.1002/anie.202418141. Epub 2024 Dec 8. Angew Chem Int Ed Engl. 2025. PMID: 39607360
-
Transition Metal-Catalyzed Regioselective Direct C-H Amidation: Interplay between Inner- and Outer-Sphere Pathways for Nitrene Cross-Coupling Reactions.Acc Chem Res. 2022 Aug 2;55(15):2123-2137. doi: 10.1021/acs.accounts.2c00283. Epub 2022 Jul 19. Acc Chem Res. 2022. PMID: 35853135
-
Aminative Suzuki-Miyaura coupling.Science. 2024 Mar;383(6686):1019-1024. doi: 10.1126/science.adl5359. Epub 2024 Feb 29. Science. 2024. PMID: 38422125
-
Davis-Beirut Reaction: Diverse Chemistries of Highly Reactive Nitroso Intermediates in Heterocycle Synthesis.Acc Chem Res. 2019 Aug 20;52(8):2256-2265. doi: 10.1021/acs.accounts.9b00220. Epub 2019 Jul 22. Acc Chem Res. 2019. PMID: 31328502 Free PMC article. Review.
-
Catalytic synthesis of azoarenes via metal-mediated nitrene coupling.Dalton Trans. 2022 Mar 22;51(12):4577-4589. doi: 10.1039/d2dt00228k. Dalton Trans. 2022. PMID: 35229862 Review.
References
-
- Denmark S. E. and Sweis R. F., Metal-catalyzed cross-coupling reactions, Wiley-VCH, Weinheim, 2004, pp. 163–216
-
- Kostas I. D., Transition metal catalyzed cross-coupling reactions, MDPI – Multidisciplinary Digital Publishing Institute, Basel, Switzerland, 2021
-
- Lei A. Liu C. Jin L. Synlett. 2010:2527–2536. doi: 10.1055/s-0030-1258802. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

