Morphology and connectivity of retinal horizontal cells in two avian species
- PMID: 40103750
- PMCID: PMC11914121
- DOI: 10.3389/fncel.2025.1558605
Morphology and connectivity of retinal horizontal cells in two avian species
Abstract
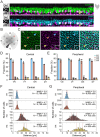
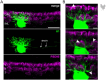
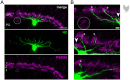
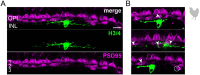
In the outer vertebrate retina, the visual signal is separated into intensity and wavelength information. In birds, seven types of photoreceptors (one rod, four single cones, and two members of the double cone) mediate signals to >20 types of second-order neurons, the bipolar cells and horizontal cells. Horizontal cells contribute to color and contrast processing by providing feedback signals to photoreceptors and feedforward signals to bipolar cells. In fish, reptiles, and amphibians they either encode intensity or show color-opponent responses. Yet, for the bird retina, the number of horizontal cell types is not fully resolved and even more importantly, the synapses between photoreceptors and horizontal cells have never been quantified for any bird species. With a combination of light microscopy and serial EM reconstructions, we found four different types of horizontal cells in two distantly related species, the domestic chicken and the European robin. In agreement with some earlier studies, we confirmed two highly abundant cell types (H1, H2) and two rare cell types (H3, H4), of which H1 is an axon-bearing cell, whereas H2-H4 are axonless. H1 cells made chemical synapses with one type of bipolar cell and an interplexiform amacrine cell at their soma. Dendritic contacts of H1-H4 cells to photoreceptors were type-specific and similar to the turtle retina, which confirms the high degree of evolutionary conservation in the vertebrate outer retina. Our data further suggests that H1 and potentially H2 cells may encode intensity, whereas H3 and H4 may represent color opponent horizontal cells which may contribute to the birds' superb color and/or high acuity vision.
Keywords: European robin; avian retina; chicken; connectomics; horizontal cells; magnetoreception; photoreceptors; vision.
Copyright © 2025 Günther, Balaji, Leberecht, Forst, Rotov, Woldt, Abdulazhanova, Mouritsen and Dedek.
Conflict of interest statement
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
Double Cones and the Diverse Connectivity of Photoreceptors and Bipolar Cells in an Avian Retina.J Neurosci. 2021 Jun 9;41(23):5015-5028. doi: 10.1523/JNEUROSCI.2495-20.2021. Epub 2021 Apr 23. J Neurosci. 2021. PMID: 33893221 Free PMC article.
-
Immunohistochemical characterization of bipolar cells in four distantly related avian species.J Comp Neurol. 2023 Mar;531(4):561-581. doi: 10.1002/cne.25443. Epub 2022 Dec 22. J Comp Neurol. 2023. PMID: 36550622
-
Synaptic organization of the outer plexiform layer of the turtle retina: an electron microscope study of serial sections.J Neurocytol. 1984 Aug;13(4):567-91. doi: 10.1007/BF01148080. J Neurocytol. 1984. PMID: 6481412
-
Distinct synaptic mechanisms create parallel S-ON and S-OFF color opponent pathways in the primate retina.Vis Neurosci. 2014 Mar;31(2):139-51. doi: 10.1017/S0952523813000230. Epub 2013 Jul 29. Vis Neurosci. 2014. PMID: 23895762 Free PMC article. Review.
-
Localization and function of dopamine in the adult vertebrate retina.Neurochem Int. 1992 Feb;20(2):139-91. doi: 10.1016/0197-0186(92)90166-o. Neurochem Int. 1992. PMID: 1304857 Review.
Cited by
-
A standardized nomenclature for the rods and cones of the vertebrate retina.PLoS Biol. 2025 May 7;23(5):e3003157. doi: 10.1371/journal.pbio.3003157. eCollection 2025 May. PLoS Biol. 2025. PMID: 40333813 Free PMC article.
-
AAV-mediated transduction of songbird retina.Front Physiol. 2025 Mar 19;16:1549585. doi: 10.3389/fphys.2025.1549585. eCollection 2025. Front Physiol. 2025. PMID: 40177359 Free PMC article.
References
-
- Ammermüller J., Kolb H. (1996). Functional architecture of the turtle retina. Prog. Retin. Eye Res. 15, 393–433. doi: 10.1016/1350-9462(96)00009-2 - DOI
LinkOut - more resources
Full Text Sources

