JAK inhibition and axial spondyloarthritis: new steps on the path to understanding pathophysiology
- PMID: 40103808
- PMCID: PMC11913702
- DOI: 10.3389/fimmu.2025.1488357
JAK inhibition and axial spondyloarthritis: new steps on the path to understanding pathophysiology
Abstract
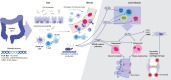
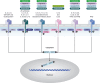
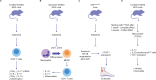
Axial spondyloarthritis (axSpA) is a chronic inflammatory disease that predominantly affects the sacroiliac joints and spine. Tumor necrosis factor (TNF) and interleukin (IL)-17A are key cytokines in disease pathogenesis and are established axSpA treatment targets. Recently, axSpA treatment options have been complemented by Janus kinase inhibitors (JAKi), which inhibit various cytokines without directly impacting TNF or IL-17 signaling. The effect of JAKi on axSpA remains under investigation: besides a JAK2-mediated (and potentially tyrosine kinase 2 [TYK2]-mediated) effect on the IL-23/IL-17 axis, emerging evidence suggests γδ T cells, type 3 innate lymphoid cells, and mucosa-associated invariant T cells, which are dependent on IL-7 and/or IL-15 and thus on JAK1, are strongly inhibited by JAKi used to treat axSpA. This review summarizes potential effects of JAKi on axSpA and shows evidence from pre-clinical/clinical studies. Greater understanding of the mechanisms of action of available treatments may improve knowledge of axSpA and pave the road for future therapies.
Keywords: Axial spondyloarthritis; JAK-STAT pathway; Janus kinase inhibitor; gut-joint axis; pathophysiology.
Copyright © 2025 Ciccia, McGonagle, Thomas, Marzo-Ortega, Martin, Yndestad and Volkov.
Conflict of interest statement
Author DM is employed by the company Pfizer Inc. Authors AY and MV were employed by Pfizer Inc. at the time of this study. DM has received honoraria and research support from AbbVie, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, MoonLake, Novartis, Pfizer Inc, and UCB. HM-O has acted as a consultant for AbbVie, Biogen, Celgene, Eli Lilly, Janssen, MoonLake, Novartis, Pfizer Inc, Takeda, and UCB, and has received grants and/or research support from Janssen, Novartis, Pfizer Inc, and UCB. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Pfizer. The funder had the following involvement in the study: Editorial assistance, under the direction of the authors, was provided by Justine Juana, BHSc, CMC Connect, a division of IPG Health Medical Communications, and was funded by Pfizer, New York, NY, USA, in accordance with Good Publication Practice (GPP 2022) guidelines (Ann Intern Med 2022;175:1298-304).
Figures
References
-
- Macfarlane GJ, Rotariu O, Jones GT, Pathan E, Dean LE. Determining factors related to poor quality of life in patients with axial spondyloarthritis: results from the British Society for Rheumatology Biologics Register (BSRBR-AS). Ann Rheum Dis. (2020) 79:202–8. doi: 10.1136/annrheumdis-2019-216143 - DOI - PubMed
-
- Nowell WB, Gavigan K, Hunter T, Malatestinic WN, Bolce RJ, Lisse JR, et al. . Treatment satisfaction and decision-making from the patient perspective in axial spondyloarthritis: real-world data from a descriptive cross-sectional survey study from the ArthritisPower registry. ACR Open Rheumatol. (2022) 4:85–94. doi: 10.1002/acr2.11365 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

