Gamma-irradiated fowl cholera vaccines formulated with different adjuvants induced antibody response and cytokine expression in chickens
- PMID: 40103817
- PMCID: PMC11914910
- DOI: 10.3389/fimmu.2025.1513443
Gamma-irradiated fowl cholera vaccines formulated with different adjuvants induced antibody response and cytokine expression in chickens
Abstract
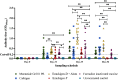
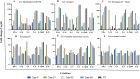
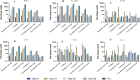
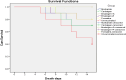
Fowl cholera is one of the most serious and economically important infectious diseases of poultry caused by Pasteurella multocida. Formalin-inactivated vaccine, administered intramuscularly, is widely used in Ethiopia with a low success rate. Gamma irradiation is an effective approach to inactivate pathogens for vaccine development. In a previous study, we reported the feasibility of developing gamma-irradiated vaccines that induced both systemic and mucosal antibody responses with complete protection against homologous lethal challenge. In the present study, we aimed to broaden our understanding of the immunogenicity of the gamma-irradiated vaccines by including peripheral blood mononuclear cells (PBMC) response analysis. A total of 156 eight-week-old fowl cholera-specific antibody negative Bovans Brown chickens were utilized in this experiment. The performances of gamma-irradiated P. multocida vaccines formulated with different adjuvants, Montanide Gel 01 PR (G-1), Carbigen® (G-2), Emulsigen-D®+aluminum hydroxide gel (G-3), and Emulsigen-p® (G-4) were evaluated in comparison with the formalin-inactivated vaccine (G-5) and unvaccinated control (G-6). Chickens received two doses of the vaccines at days 0 and 21. Sera, tracheal, and crop lavage were collected at days 0, 21, 35, and 56 to assess IgG and IgA levels using indirect and sandwich ELISA, respectively. PBMC proliferation was compared between vaccinated and unvaccinated controls. In addition, vaccination-induced expression of cytokine genes was analyzed in PBMC using qPCR. Chickens were challenged with 2.5x107 CFU/ml of P. multocida biotype A intramuscularly one day after day-56 sampling. Significant serum IgG titers were detected three weeks after primary vaccination in G1, G3, and G5. IgG titer substantially increased in all vaccinated groups two weeks post-booster dose. IgA response was induced by gamma-irradiated vaccines but not formalin-inactivated vaccines. Only PBMC from vaccinated chickens proliferated in response to re-stimulation with P. multocida antigen, indicating vaccine-specific priming. Interestingly, gamma-irradiated vaccines resulted in a higher fold change in mRNA transcripts of IFN-γ (>1000-fold change) IL-6 (>500-fold change), and IL-12p40 (>200-fold change), which are hallmarks of a Th1 dominant response, which is essential to combat intracellular infection. Lastly, the candidate vaccines demonstrated various levels of protection, with Emulsigen-D® containing vaccine rendering complete protection against homologous lethal challenge. In conclusion, gamma-irradiated vaccines can induce broad immune responses, humoral and cellular, and protect against severe outcome of fowl cholera. Therefore, this study has contributed to growing knowledge on the immunogenicity and efficacy of gamma-irradiated vaccines and has shown the potential of such a vaccine platform for field application in extensive as well as intensive farm settings.
Keywords: P. multocida; adjuvants; chicken; cytokines; fowl cholera; gamma-irradiated vaccines; humoral immunity; mucosal immunity.
Copyright © 2025 Belay, Bitew, Ibrahim, Dessalegn, Abey, Dejene, Birhan, Duffera, Asefa, Tesfaw, Abayneh, Sherefa, W/Medhin, Tesfaye, Tuki, Gelaye, Kangethe, Wijewardana and Bravo De Rueda.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bitew M, Belihu K, Gebre-Egziabher B, Kyule M. Development and efficacy trial of inactivated Fowl cholera vaccine using local isolates of Pasteurella multocida. Ethiop Vet J. (2009) 13:81–98.
-
- Akhtar M, Rahman M, Ara M, Rahman M, Nazir K, Ahmed S, et al. . Isolation of Pasteurella multocida from chickens, preparation of formalin killed fowl cholera vaccine, and determination of efficacy in experimental chickens. J Adv Vet Ani Res. (2016) 3:45–50. doi: 10.5455/javar.2016.c130 - DOI
-
- Wubet W, Bitew M, Mamo G, Gelaye E, Tesfaw L, Sori H, et al. . Evaluation of inactivated vaccine against fowl cholera developed from local isolates of Pasteurella multocida in Ethiopia. Afr J Microbiol Res. (2019) 13:500–9. doi: 10.5897/AJMR2019.9096 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

