Technological breakthroughs and advancements in the application of mRNA vaccines: a comprehensive exploration and future prospects
- PMID: 40103818
- PMCID: PMC11913674
- DOI: 10.3389/fimmu.2025.1524317
Technological breakthroughs and advancements in the application of mRNA vaccines: a comprehensive exploration and future prospects
Abstract
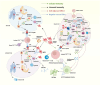
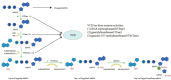
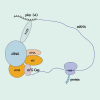
mRNA vaccines utilize single-stranded linear DNA as a template for in vitro transcription. The mRNA is introduced into the cytoplasm via the corresponding delivery system to express the target protein, which then performs its relevant biological function. mRNA vaccines are beneficial in various fields, including cancer vaccines, infectious disease vaccines, protein replacement therapy, and treatment of rare diseases. They offer advantages such as a simple manufacturing process, a quick development cycle, and ease of industrialization. Additionally, mRNA vaccines afford flexibility in adjusting antigen designs and combining sequences of multiple variants, thereby addressing the issue of frequent mutations in pathogenic microorganisms. This paper aims to provide an extensive review of the global development and current research status of mRNA vaccines, with a focus on immunogenicity, classification, design, delivery vector development, stability, and biomedical application. Moreover, the study highlights current challenges and offers insights into future directions for development.
Keywords: biomedical application; classification; delivery vector development; design; immunogenicity; mRNA vaccines; stability.
Copyright © 2025 Wei, Zhang, Wang, Xue, Dang and Zhai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
mRNA Vaccines: Design Principles, Mechanisms, and Manufacturing-Insights From COVID-19 as a Model for Combating Infectious Diseases.Biotechnol J. 2025 Feb;20(2):e202400596. doi: 10.1002/biot.202400596. Biotechnol J. 2025. PMID: 39989260 Review.
-
Computational biology and artificial intelligence in mRNA vaccine design for cancer immunotherapy.Front Cell Infect Microbiol. 2025 Jan 20;14:1501010. doi: 10.3389/fcimb.2024.1501010. eCollection 2024. Front Cell Infect Microbiol. 2025. PMID: 39902185 Free PMC article. Review.
-
RNA vaccines: The dawn of a new age for tuberculosis?Hum Vaccin Immunother. 2025 Dec;21(1):2469333. doi: 10.1080/21645515.2025.2469333. Epub 2025 Feb 27. Hum Vaccin Immunother. 2025. PMID: 40013818 Free PMC article. Review.
-
Current state of, prospects for, and obstacles to mRNA vaccine development.Drug Discov Today. 2023 Feb;28(2):103458. doi: 10.1016/j.drudis.2022.103458. Epub 2022 Nov 24. Drug Discov Today. 2023. PMID: 36427779 Review.
-
mRNA vaccine sequence and structure design and optimization: Advances and challenges.J Biol Chem. 2025 Jan;301(1):108015. doi: 10.1016/j.jbc.2024.108015. Epub 2024 Nov 26. J Biol Chem. 2025. PMID: 39608721 Free PMC article. Review.
Cited by
-
Signal Peptides: From Molecular Mechanisms to Applications in Protein and Vaccine Engineering.Biomolecules. 2025 Jun 18;15(6):897. doi: 10.3390/biom15060897. Biomolecules. 2025. PMID: 40563537 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

