The N-formyl peptide receptors: much more than chemoattractant receptors. Relevance in health and disease
- PMID: 40103822
- PMCID: PMC11913705
- DOI: 10.3389/fimmu.2025.1568629
The N-formyl peptide receptors: much more than chemoattractant receptors. Relevance in health and disease
Abstract
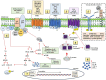
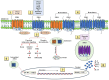
Pattern Recognition Receptors (PRRs) are a superfamily of receptors that detect molecular structures typical for pathogens and damaged cells and play a crucial role in the proper function of the innate immune system. A particular subgroup of membrane-bound PRRs is represented by the N-formyl peptide receptors (FPRs) that consist of transmembrane G-protein coupled receptors involved in inflammatory responses. FPRs were initially described in immune cells as transducers of chemotactic signals in phagocytes that react to tissue injury. Subsequently, FPRs were also identified in a wide variety of cell types, including cancer cells. Beyond broad cellular distribution, FPRs are also characterized by the ability to bind a variety of ligands with different chemical and biological properties, ranging from natural peptides to synthetic compounds. The binding of FPRs to specific agonists induces a cascade of functional biological events, such as cell proliferation, migration, angiogenesis, and oxidative stress. From all this evidence, it becomes clear that FPRs are multifaceted receptors involved in several pathophysiological processes associated with inflammation. In this review, we provide a comprehensive molecular description of structure-function relationship of FPRs and their pivotal role in the host defense, highlighting the regulatory functions in both the initiation and resolution of inflammation. In addition to their activity as PRRs during innate immune response, we focus on their involvement in pathological conditions, including chronic inflammatory disease, neurodegenerative disorders, and cancer, with special emphasis on FPR targeting as promising therapeutic strategies in the era of precision medicine.
Keywords: G-protein coupled receptors; N-formyl peptide receptors; chemoattractant receptors; inflammation; receptor structure-function.
Copyright © 2025 Napolitano and Montuori.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Regulation of inflammation by members of the formyl-peptide receptor family.J Autoimmun. 2017 Dec;85:64-77. doi: 10.1016/j.jaut.2017.06.012. Epub 2017 Jul 6. J Autoimmun. 2017. PMID: 28689639 Free PMC article. Review.
-
The Formyl Peptide Receptors: Diversity of Ligands and Mechanism for Recognition.Molecules. 2017 Mar 13;22(3):455. doi: 10.3390/molecules22030455. Molecules. 2017. PMID: 28335409 Free PMC article. Review.
-
Formyl Peptide Receptors in Cellular Differentiation and Inflammatory Diseases.J Cell Biochem. 2017 Jun;118(6):1300-1307. doi: 10.1002/jcb.25877. Epub 2017 Mar 2. J Cell Biochem. 2017. PMID: 28075050 Review.
-
Biased perspectives on formyl peptide receptors.Biochim Biophys Acta Mol Cell Res. 2019 Feb;1866(2):305-316. doi: 10.1016/j.bbamcr.2018.11.015. Epub 2018 Dec 4. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30521870 Review.
-
The Role of Formyl Peptide Receptors in Permanent and Low-Grade Inflammation: Helicobacter pylori Infection as a Model.Int J Mol Sci. 2021 Apr 2;22(7):3706. doi: 10.3390/ijms22073706. Int J Mol Sci. 2021. PMID: 33918194 Free PMC article. Review.
Cited by
-
Bioactive Compounds as Modulators of N-Formyl Peptide Signaling in Chronic Diseases.Molecules. 2025 Jul 16;30(14):2981. doi: 10.3390/molecules30142981. Molecules. 2025. PMID: 40733247 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

