Persistent innate immune dysfunction and ZIKV replication in the gastrointestinal tract during SIV infection in pigtail macaques
- PMID: 40103823
- PMCID: PMC11913663
- DOI: 10.3389/fimmu.2025.1535807
Persistent innate immune dysfunction and ZIKV replication in the gastrointestinal tract during SIV infection in pigtail macaques
Abstract
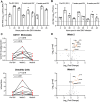
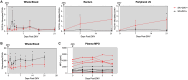
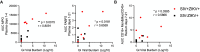
Mosquito-borne flaviviruses, including dengue (DENV) and Zika (ZIKV) viruses, have caused widespread epidemics in areas with high HIV prevalence, partly due to the expanded geographic range of arthropod vectors. Despite the occurrence of large flavivirus outbreaks in areas with high HIV prevalence, little is known about the effects of flavivirus infection in people living with HIV (PLWH). Here, we use a pigtail macaque model of HIV/AIDS to investigate the impact of simian immunodeficiency virus (SIV)-induced immunosuppression on ZIKV replication and pathogenesis. During acute SIV infection, peripheral ZIKV cellular targets expanded and innate immune activation increased. In vitro, peripheral blood mononuclear cells (PBMC) from SIV infected pigtail macaques were less permissive to ZIKV infection. In vivo, ZIKV viremia was delayed and ZIKV was more persistent in the gastrointestinal tissues of SIV-ZIKV co-infected animals. This persistence was associated with changes in innate cellular (monocytes, neutrophils) recruitment to the blood and tissues, reduced anti-ZIKV immunity, and sustained expression of peripheral inflammatory and innate immune genes. Collectively, these findings uniquely suggest that untreated SIV infection may promote inflammatory cellular innate responses and create a state of persistent immune activation that contributes to prolonged ZIKV viremia and persistence in the gastrointestinal tract. Furthermore, these results suggest that PLWH and other immunocompromised individuals could be at higher risk for prolonged ZIKV infection, potentially extending the window of ZIKV transmission. These insights highlight the importance of including PLWH in strategies for deploying vaccines and treatments against ZIKV.
Keywords: Zika virus; co-infection; innate immunity; nonhuman primate; simian immunodeficiency virus (SIV).
Copyright © 2025 Tisoncik-Go, Lewis, Whitmore, Voss, Niemeyer, Dai, Kim, Hubbell, Iwayama, Ahrens, Wangari, Murnane, Edlefsen, Guerriero, Gale, Fuller and O’Connor.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Update of
-
Chronic innate immune impairment and ZIKV persistence in the gastrointestinal tract during SIV infection in pigtail macaques.bioRxiv [Preprint]. 2024 Aug 23:2024.08.23.609309. doi: 10.1101/2024.08.23.609309. bioRxiv. 2024. Update in: Front Immunol. 2025 Mar 04;16:1535807. doi: 10.3389/fimmu.2025.1535807. PMID: 39229223 Free PMC article. Updated. Preprint.
Similar articles
-
Chronic innate immune impairment and ZIKV persistence in the gastrointestinal tract during SIV infection in pigtail macaques.bioRxiv [Preprint]. 2024 Aug 23:2024.08.23.609309. doi: 10.1101/2024.08.23.609309. bioRxiv. 2024. Update in: Front Immunol. 2025 Mar 04;16:1535807. doi: 10.3389/fimmu.2025.1535807. PMID: 39229223 Free PMC article. Updated. Preprint.
-
Simian Immunodeficiency Virus Infection of Rhesus Macaques Results in Delayed Zika Virus Clearance.mBio. 2019 Dec 3;10(6):e02790-19. doi: 10.1128/mBio.02790-19. mBio. 2019. PMID: 31796542 Free PMC article.
-
Comparative evaluation of simian, simian-human, and human immunodeficiency virus infections in the pigtail macaque (Macaca nemestrina) model.AIDS Res Hum Retroviruses. 2006 Jun;22(6):580-8. doi: 10.1089/aid.2006.22.580. AIDS Res Hum Retroviruses. 2006. PMID: 16796533
-
A case for innate immune effector mechanisms as contributors to disease resistance in SIV-infected sooty mangabeys.Curr HIV Res. 2009 Jan;7(1):12-22. doi: 10.2174/157016209787048465. Curr HIV Res. 2009. PMID: 19149550 Review.
-
Generalized immune activation and innate immune responses in simian immunodeficiency virus infection.Curr Opin HIV AIDS. 2011 Sep;6(5):411-8. doi: 10.1097/COH.0b013e3283499cf6. Curr Opin HIV AIDS. 2011. PMID: 21743324 Free PMC article. Review.
References
-
- Organization WHO . Vector-borne diseases (2024). Available online at: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (Accessed September 26, 2024).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

