Tumor-derived extracellular vesicles: key drivers of immunomodulation in breast cancer
- PMID: 40103824
- PMCID: PMC11914124
- DOI: 10.3389/fimmu.2025.1548535
Tumor-derived extracellular vesicles: key drivers of immunomodulation in breast cancer
Abstract
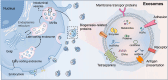
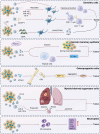
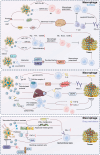
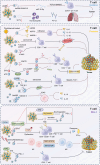
Breast cancer (BC) remains a significant global health challenge characterized by its heterogeneity and treatment complexities. Extracellular vesicles (EVs) are small membranous particles released by cells, facilitating intercellular communication by transporting bioactive molecules such as proteins, lipids, and nucleic acids. Tumor-derived EVs have emerged as pivotal regulators in the tumor microenvironment (TME) and drivers of BC progression. These EVs carry diverse cargoes of bioactive molecules, influencing critical processes such as immune modulation, angiogenesis, and metastasis. By altering the behaviors of immune cells including macrophages, dendritic cells, and T cells, tumor-derived EVs contribute to immune evasion and tumor growth. Furthermore, Tumor-derived EVs play a role in mediating drug resistance, impacting the effectiveness of therapeutic interventions. Understanding the multifaceted roles of BC tumor-derived EVs is essential for the development of innovative therapeutic strategies. Targeting pathways mediated by EVs holds promise for enhancing the efficacy of cancer treatments and improving patient outcomes. This comprehensive review provides insights into the intricate interactions of tumor-derived EVs in immune modulation and BC progression, highlighting potential therapeutic targets and avenues for novel cancer therapies.
Keywords: T cells; breast cancer; extracellular vesicles; immune regulation; macrophages.
Copyright © 2025 Li, Yu, Rao and Cheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Extracellular vesicles in tumor immunity: mechanisms and novel insights.Mol Cancer. 2025 Feb 14;24(1):45. doi: 10.1186/s12943-025-02233-w. Mol Cancer. 2025. PMID: 39953480 Free PMC article. Review.
-
Unlocking the Secrets of Extracellular Vesicles: Orchestrating Tumor Microenvironment Dynamics in Metastasis, Drug Resistance, and Immune Evasion.J Cancer. 2024 Oct 14;15(19):6383-6415. doi: 10.7150/jca.98426. eCollection 2024. J Cancer. 2024. PMID: 39513123 Free PMC article. Review.
-
Extracellular vesicles in cancer´s communication: messages we can read and how to answer.Mol Cancer. 2025 Mar 19;24(1):86. doi: 10.1186/s12943-025-02282-1. Mol Cancer. 2025. PMID: 40108630 Free PMC article. Review.
-
Protrusion-Derived Extracellular Vesicles (PD-EVs) and Their Diverse Origins: Key Players in Cellular Communication, Cancer Progression, and T Cell Modulation.Biol Cell. 2025 Jun;117(6):e70018. doi: 10.1111/boc.70018. Biol Cell. 2025. PMID: 40500981 Free PMC article. Review.
-
Tumor-derived extracellular vesicles: Hijacking T cell function through exhaustion.Pathol Res Pract. 2025 May;269:155948. doi: 10.1016/j.prp.2025.155948. Epub 2025 Mar 29. Pathol Res Pract. 2025. PMID: 40168777 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

