Extreme signal amplitude events in neuromagnetic oscillations reveal brain aging processing across adulthood
- PMID: 40103930
- PMCID: PMC11914120
- DOI: 10.3389/fnagi.2025.1498400
Extreme signal amplitude events in neuromagnetic oscillations reveal brain aging processing across adulthood
Abstract
Introduction: Neurophysiological activity, as noninvasively captured by electro- and magnetoencephalography (EEG and MEG), demonstrates complex temporal fluctuations approximated by typical variations around the mean values and rare events with large amplitude. The statistical properties of these extreme and rare events in neurodynamics may reflect the limits or capacity of the brain as a complex system in information processing. However, the exact role of these extreme neurodynamic events in ageing, and their spectral and spatial patterns remain elusive. Our study hypothesized that ageing would be associated with frequency specific alterations in the brain's tendency to synchronize large ensembles of neurons and to produce extreme events.
Methods: To identify spatio-spectral patterns of these age-related changes in extreme neurodynamics, we examined resting-state MEG recordings from a large cohort of adults (n = 645), aged 18 to 89. We characterized extreme neurodynamics by computing sample skewness and kurtosis, and used Partial Least Squares to test for differences across age groups.
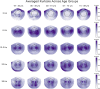
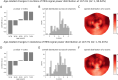
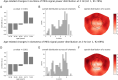
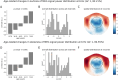
Results: Our findings revealed that each canonical frequency, from theta to lower gamma, displayed unique spatial patterns of either age-related increases, decreases, or both in the brain's tendency to produce extreme neuromagnetic events.
Discussion: Our study introduces a novel neuroimaging framework for understanding ageing through the extreme and rare events of the neurophysiological activity, offering more sensitivity than typical comparative approaches.
Keywords: ageing; brain rhythms; extreme values; heavy tail distributions; magnetoencephalography; neuronal avalanches; skewed distributions; temporal variability.
Copyright © 2025 Vakorin, Liaqat, Doesburg and Moreno.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
A multivariate, spatiotemporal analysis of electromagnetic time-frequency data of recognition memory.Neuroimage. 2003 Feb;18(2):185-97. doi: 10.1016/s1053-8119(02)00031-9. Neuroimage. 2003. PMID: 12595175
-
Low-frequency connectivity is associated with mild traumatic brain injury.Neuroimage Clin. 2015 Mar 3;7:611-21. doi: 10.1016/j.nicl.2015.02.020. eCollection 2015. Neuroimage Clin. 2015. PMID: 25844315 Free PMC article.
-
Quasicriticality explains variability of human neural dynamics across life span.Front Comput Neurosci. 2022 Nov 30;16:1037550. doi: 10.3389/fncom.2022.1037550. eCollection 2022. Front Comput Neurosci. 2022. PMID: 36532868 Free PMC article.
-
Source analysis of EEG oscillations using high-resolution EEG and MEG.Prog Brain Res. 2006;159:29-42. doi: 10.1016/S0079-6123(06)59003-X. Prog Brain Res. 2006. PMID: 17071222 Free PMC article. Review.
-
Adult lifespan trajectories of neuromagnetic signals and interrelations with cortical thickness.Neuroimage. 2023 Sep;278:120275. doi: 10.1016/j.neuroimage.2023.120275. Epub 2023 Jul 13. Neuroimage. 2023. PMID: 37451375 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

