Sex-related differences in genetically determined Alzheimer's disease
- PMID: 40103931
- PMCID: PMC11913828
- DOI: 10.3389/fnagi.2025.1522434
Sex-related differences in genetically determined Alzheimer's disease
Abstract
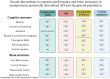
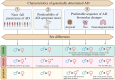
We reviewed the literature on sex differences in genetically determined Alzheimer's disease (AD), focusing on autosomal dominant AD (ADAD), Down syndrome-associated AD (DSAD), and APOE4 homozygosity, particularly regarding disease penetrance, symptom onset and clinical progression, and trajectories for markers of amyloidosis (A), tau pathology (T) and neurodegeneration (N). Data suggests that sex differences in disease penetrance, symptom onset, and AT(N) biomarker trajectories are typically subtle for genetically determined AD populations. Noteworthy exceptions, such as increased neurodegeneration in later stages of the disease in females while similar cognitive outcomes, suggest a potential differential cognitive reserve that warrants further investigation. Additionally, the interaction between APOE genotype and sex reveals complex and multifaceted effects in DSAD, with potential implications for ADAD that remain underexplored. The smaller sex differences observed compared to sporadic AD offer insights into the different underlying disease mechanisms in genetically determined AD populations. Future research should prioritize sex-specific investigations in genetically determined AD, focusing on refining methodologies. This includes prioritizing longitudinal designs, adjustment for key confounders, and adherence to sex-specific guidelines.
Keywords: AT(N) biomarker trajectories in Alzheimer’s disease (amyloid, tau and neurodegeneration biomarkers); apolipoprotein E epsilon 4 (APOE4) homozygosity; autosomal dominant Alzheimer’s disease (ADAD); disease penetrance in Alzheimer’s disease; down syndrome-associated Alzheimer’s disease (DS-AD); genetically determined Alzheimer’s disease; sex differences in Alzheimer’s disease; symptom onset in Alzheimer’s disease.
Copyright © 2025 Del Hoyo Soriano, Wagemann, Bejanin, Levin and Fortea.
Conflict of interest statement
JF has received personal fees for service on advisory boards, adjudication committees, or speaker honoraria from AC Immune, Adamed, Alzheon, Biogen, Eisai, Esteve, Fujirebio, Ionis, Laboratorios Carnot, Life Molecular Imaging, Lilly, Lundbeck, Perha, and Roche, outside the submitted work. JF also holds a patent for markers of synaptopathy in neurodegenerative disease (licensed to Adx, EPI8382175.0). JL reports speaker fees from Bayer Vital, Biogen, EISAI, TEVA, Zambon, Esteve, Merck and Roche, consulting fees from Axon Neuroscience, EISAI and Biogen, author fees from Thieme medical publishers and W. Kohlhammer GmbH medical publishers and is inventor in a patent “Oral Phenylbutyrate for Treatment of Human 4-Repeat Tauopathies” (PCT/EP2024/053388) filed by LMU Munich. In addition, JL reports compensation for serving as chief medical officer for MODAG GmbH is beneficiary of the phantom share program of MODAG GmbH and is inventor in a patent “Pharmaceutical Composition and Methods of Use” (EP 22 159 408.8) filed by MODAG GmbH, all activities outside the submitted work. The other authors report no relevant disclosures. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor SG declared a past collaboration with the author JF.
Figures

Similar articles
-
Cerebral amyloid angiopathy in Down syndrome and sporadic and autosomal-dominant Alzheimer's disease.Alzheimers Dement. 2017 Nov;13(11):1251-1260. doi: 10.1016/j.jalz.2017.03.007. Epub 2017 Apr 29. Alzheimers Dement. 2017. PMID: 28463681 Free PMC article.
-
ApoE4 effects on automated diagnostic classifiers for mild cognitive impairment and Alzheimer's disease.Neuroimage Clin. 2014 Jan 4;4:461-72. doi: 10.1016/j.nicl.2013.12.012. eCollection 2014. Neuroimage Clin. 2014. PMID: 24634832 Free PMC article.
-
APOE4 homozygozity represents a distinct genetic form of Alzheimer's disease.Nat Med. 2024 May;30(5):1284-1291. doi: 10.1038/s41591-024-02931-w. Epub 2024 May 6. Nat Med. 2024. PMID: 38710950
-
Alzheimer's Disease in Down Syndrome: Progress in the Design and Conduct of Drug Prevention Trials.CNS Drugs. 2020 Aug;34(8):785-794. doi: 10.1007/s40263-020-00740-6. CNS Drugs. 2020. PMID: 32506291 Free PMC article. Review.
-
Tau PET Imaging for Staging of Alzheimer's Disease in Down Syndrome.Dev Neurobiol. 2019 Jul;79(7):711-715. doi: 10.1002/dneu.22658. Epub 2018 Dec 16. Dev Neurobiol. 2019. PMID: 30536948 Review.
Cited by
-
Age and sex are associated with Alzheimer's disease neuropathology in Down syndrome.Alzheimers Dement. 2025 Jul;21(7):e70408. doi: 10.1002/alz.70408. Alzheimers Dement. 2025. PMID: 40673442 Free PMC article.
-
Mitochondrial Imbalance in Down Syndrome: A Driver of Accelerated Brain Aging?Aging Dis. 2025 Apr 6;16(5):2674-2694. doi: 10.14336/AD.2025.0189. Aging Dis. 2025. PMID: 40249934 Free PMC article. Review.
-
Special Issue: "New Trends in Alzheimer's Disease Research: From Molecular Mechanisms to Therapeutics: 2nd Edition".Int J Mol Sci. 2025 Jul 25;26(15):7175. doi: 10.3390/ijms26157175. Int J Mol Sci. 2025. PMID: 40806308 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

