Fe3O4@SiO2-BD-DADB-COF is proposed as a novel magnetic covalent organic framework for the determination and extraction of 15 macrolide antibiotics in water and honey
- PMID: 40103973
- PMCID: PMC11913072
- DOI: 10.1039/d5ra00080g
Fe3O4@SiO2-BD-DADB-COF is proposed as a novel magnetic covalent organic framework for the determination and extraction of 15 macrolide antibiotics in water and honey
Abstract
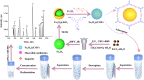
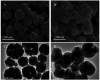
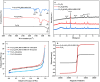
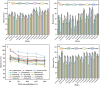
In our research, an emerging magnetic covalent organic framework (Fe3O4@SiO2-BD-DADB-COF) was formulated through Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), etc. Several parameters affecting the extraction process were refined. Accordingly, a novel method of determining 15 macrolides (MALs) in honey and water was established through the Fe3O4@SiO2-BD-DADB-COF as a magnetic solid-phase extraction (MSPE) adsorbent and ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). In consequence, the standard curves for the 15 MALs exhibited an exceptional linearity from 0.1 to 200 μg L-1, and the correlation coefficients (R 2) varied from 0.9990 to 0.9999. The recoveries fell between 70.01% and 115.56%, with the relative standard deviations (RSDs) being below 9.93% (n = 5). The detection limits reached 0.001-0.075 μg L-1 with the quantification limits being 0.004-0.228 μg L-1. Ultimately, the method was excellently applied to the analysis of MALs in honey and water.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Cañadas R. Martínez R. M. G. González G. P. Hernando P. F. Polymer. 2022;249:124843.
-
- García-Mayor M. A. Gallego-Picó A. Garcinuño R. M. Fernández-Hernando P. Durand-Alegría J. S. Food Chem. 2012;134:553–558.
-
- Myers A. G. Clark R. B. Acc. Chem. Res. 2021;54:1635–1645. - PubMed
LinkOut - more resources
Full Text Sources

